Professional Documents
Culture Documents
Histidine
Histidine
Uploaded by
Partha Sengupta0 ratings0% found this document useful (0 votes)
7 views1 pageHistidine is an amino acid that acts as a general base in converting malate to oxaloacetate. It abstracts a proton from malate, existing in its neutral form before transferring hydrogen. Histidine also contains an imidazole ring in its side chain, which has a pKa value of around 7.0, so both acid and base forms are present at physiological pH due to resonance that allows a hydrogen atom to change positions between ring nitrogens.
Original Description:
Properties of Histidine
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentHistidine is an amino acid that acts as a general base in converting malate to oxaloacetate. It abstracts a proton from malate, existing in its neutral form before transferring hydrogen. Histidine also contains an imidazole ring in its side chain, which has a pKa value of around 7.0, so both acid and base forms are present at physiological pH due to resonance that allows a hydrogen atom to change positions between ring nitrogens.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
0 ratings0% found this document useful (0 votes)
7 views1 pageHistidine
Histidine
Uploaded by
Partha SenguptaHistidine is an amino acid that acts as a general base in converting malate to oxaloacetate. It abstracts a proton from malate, existing in its neutral form before transferring hydrogen. Histidine also contains an imidazole ring in its side chain, which has a pKa value of around 7.0, so both acid and base forms are present at physiological pH due to resonance that allows a hydrogen atom to change positions between ring nitrogens.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
You are on page 1of 1
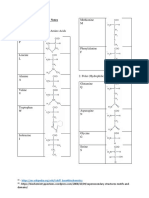
Histidine, An intersteing amino acid
Histidine acts as a general base in the conversion of malate to oxaloacetate. Histidine abstracts a proton from the 0H group of malate ion. In this case before the transfer of hydrogen Histidine is in its neutral ion. Another impetrating fact regarding Histidine is that its side chain contan imidazole ring. The pKa value for the ring is approximately 7.0, so at physiological pH, both the acid and base forms are present. In this case ai important resonance phenomenon took place, where the H atom bonded to N1 nitrogen changes its position to N3 nitrogen. Thus either of the two ring nitrogen can release a proton (H+) to produce the conjugate base.
AN(1)H AN(3)H
AN- + H+
You might also like
- Biochemical Energetics: Biochemistry of MetabolismDocument36 pagesBiochemical Energetics: Biochemistry of MetabolismDozdiNo ratings yet
- Nature - White - 2011 (Desaturacions) PDFDocument7 pagesNature - White - 2011 (Desaturacions) PDFCarlotaNo ratings yet
- Medicosis Perfectionalis Acid-Base 01.Acid-Base IntroductionDocument40 pagesMedicosis Perfectionalis Acid-Base 01.Acid-Base IntroductionMujtaba JawadNo ratings yet
- 8 Fa13Document40 pages8 Fa13FlowerNo ratings yet
- Drug Metabolism: Dr. Amal BelaidDocument28 pagesDrug Metabolism: Dr. Amal BelaidMustafa RihanNo ratings yet
- Acid - Base Blended Lesson CRDFDocument26 pagesAcid - Base Blended Lesson CRDFmypenNo ratings yet
- MCAT Amino Acids and Protein StructuresDocument5 pagesMCAT Amino Acids and Protein StructuresNnenna UjahNo ratings yet
- Curs7 EnglDocument43 pagesCurs7 Englpisabela16No ratings yet
- LifeScience I Week 1 Topic 2Document28 pagesLifeScience I Week 1 Topic 2nsemn1234No ratings yet
- 3a.protein Structure PartA CompleteDocument54 pages3a.protein Structure PartA Completeazyhuang77No ratings yet
- Medch400 Pka33Document39 pagesMedch400 Pka33서한진No ratings yet
- Coordination Complexes in Biological Systems: DR Karimah KassimDocument21 pagesCoordination Complexes in Biological Systems: DR Karimah Kassimapi-386303659No ratings yet
- Aldehydes Notes 27 May 2023Document7 pagesAldehydes Notes 27 May 2023Aafia AlamNo ratings yet
- Water and Acid-Base System: By: Dr. Mohd Fakharul ZamanDocument35 pagesWater and Acid-Base System: By: Dr. Mohd Fakharul ZamanAbdul Ashraf RasidNo ratings yet
- Acid Base Equetion SolDocument14 pagesAcid Base Equetion Solvinod bhanaNo ratings yet
- CHAPTER25 HeterocyclesDocument44 pagesCHAPTER25 HeterocyclesRiyanto WidodoNo ratings yet
- Proteins,: Sugars, LipidsDocument23 pagesProteins,: Sugars, LipidsBen BarberianNo ratings yet
- Chapter 19 Aminoacids and ProteinsDocument28 pagesChapter 19 Aminoacids and ProteinsAbdo MohdyNo ratings yet
- Application of Acid Base Titrations-1Document15 pagesApplication of Acid Base Titrations-1Adrian ChombaNo ratings yet
- PH and BuffersDocument18 pagesPH and BuffersXolane IsaacNo ratings yet
- FF - MC LECTURE PPT 2Document18 pagesFF - MC LECTURE PPT 2Dila AprilliaNo ratings yet
- Amino Acids and PeptidesDocument38 pagesAmino Acids and PeptidesrizwanamalikNo ratings yet
- Lecture 32 - TC - 10.11.23Document48 pagesLecture 32 - TC - 10.11.23yakkalivivekNo ratings yet
- Biochemical Energetics: Peranan Atp BioenergetikaDocument23 pagesBiochemical Energetics: Peranan Atp BioenergetikaHerryNo ratings yet
- 8 ACIDS Bases Buffers 09Document4 pages8 ACIDS Bases Buffers 09Sirine AjourNo ratings yet
- Fundamentals of Acids, Bases & Ionic Equilibrium: The KeyDocument23 pagesFundamentals of Acids, Bases & Ionic Equilibrium: The KeySachin KumarNo ratings yet
- Fused-Ring Heterocyclic ChemistryDocument31 pagesFused-Ring Heterocyclic ChemistrySiddarth PalletiNo ratings yet
- Lecture 4 Reactions of Aldehydes and KetonesDocument28 pagesLecture 4 Reactions of Aldehydes and KetonesKoki KingNo ratings yet
- PHARM4515 8 (Antihistamines)Document45 pagesPHARM4515 8 (Antihistamines)Street Pasutri RolferNo ratings yet
- Chapter 2 - Bronsted-Lowry TheoryDocument19 pagesChapter 2 - Bronsted-Lowry TheoryAcidri AbdulkarimNo ratings yet
- Interpretation of Arterial Blood Gases 2009 Surgery OxfordDocument5 pagesInterpretation of Arterial Blood Gases 2009 Surgery OxfordPedro GasparNo ratings yet
- Bmri2014 695281Document16 pagesBmri2014 695281Ica PurnamasariNo ratings yet
- Inorganic Chemistry: The Peroxo Complexes of TitaniumDocument10 pagesInorganic Chemistry: The Peroxo Complexes of TitaniumSandipan SahaNo ratings yet
- Book ProblemsDocument27 pagesBook Problemsعلي محمد عبد العال عبد اللهNo ratings yet
- D. Glucose + ATPDocument10 pagesD. Glucose + ATPFareeda BhattiNo ratings yet
- Medicinal Chemistry (Basic Principles) : 24 November 2010 Andrej Boháč, Milan RemkoDocument49 pagesMedicinal Chemistry (Basic Principles) : 24 November 2010 Andrej Boháč, Milan RemkoSiddarth PalletiNo ratings yet
- Antihistamines: Student Learning GoalsDocument45 pagesAntihistamines: Student Learning GoalsDaniel WangNo ratings yet
- Chem 145 Notes PDFDocument12 pagesChem 145 Notes PDFjlngnNo ratings yet
- Acids and BasesDocument7 pagesAcids and BasesNurliana RoslanNo ratings yet
- Weak Acid Strong Base TitrationDocument10 pagesWeak Acid Strong Base Titrationzb8No ratings yet
- Protein Degradation: Molecular Biochemistry IIDocument44 pagesProtein Degradation: Molecular Biochemistry IIT.K.RAJANo ratings yet
- Cellular Respiration Gulf Coast 2013 1Document14 pagesCellular Respiration Gulf Coast 2013 1hamnababar98No ratings yet
- GlycolosisDocument49 pagesGlycolosismishraravikumar428No ratings yet
- Netbook BuferDocument12 pagesNetbook Bufernur SehasariNo ratings yet
- Acid - Base Reaction Lecture 2Document22 pagesAcid - Base Reaction Lecture 2ghkdd843No ratings yet
- CHAPTER 3 - Concept of Acid-Base NeutralizationDocument49 pagesCHAPTER 3 - Concept of Acid-Base NeutralizationRichie BobbyNo ratings yet
- Curs7 EnglDocument43 pagesCurs7 EnglMahmoud מחמוד Abu ObiedNo ratings yet
- Lehninger of Principles of Biochemistry: David L. Nelson and Michael M. Cox 6 EditionDocument30 pagesLehninger of Principles of Biochemistry: David L. Nelson and Michael M. Cox 6 Editionarrigo sacchiNo ratings yet
- Acids and Bases - 5Document30 pagesAcids and Bases - 5Allen SodaNo ratings yet
- Chapter 2 Proteins (2022)Document174 pagesChapter 2 Proteins (2022)abrahamnorman56No ratings yet
- Indoles and IsoindolesDocument16 pagesIndoles and IsoindolesSylvester AsareNo ratings yet
- Universidad Nacional Mayor de San Marcos: Año de La Diversificación Productiva y Del Fortalecimiento de La EducaciónDocument8 pagesUniversidad Nacional Mayor de San Marcos: Año de La Diversificación Productiva y Del Fortalecimiento de La EducaciónAnselma PardoNo ratings yet
- Nucleophilic Substitution and Elimination ReactionsDocument51 pagesNucleophilic Substitution and Elimination ReactionsImam Syafi'iNo ratings yet
- Edexcel Chemistry A-Level: Topic 12: Acid-Base EquilibriaDocument11 pagesEdexcel Chemistry A-Level: Topic 12: Acid-Base EquilibriaLulwa KhaskiehNo ratings yet
- FF - MC Lecture PPT 2.en - IdDocument18 pagesFF - MC Lecture PPT 2.en - IdRegina SyafinatullahNo ratings yet
- Chap 04 Acids and BasesDocument22 pagesChap 04 Acids and BasesavNo ratings yet
- Jurnal KimiaaaaaaaDocument19 pagesJurnal KimiaaaaaaaZoom123No ratings yet
- Free Energy of A ReactionDocument38 pagesFree Energy of A Reactionpunkvijay@gmail.comNo ratings yet
- Heck ReactionDocument5 pagesHeck ReactionRiyAz HussAinNo ratings yet
- Brief History of Atomic TheoryDocument21 pagesBrief History of Atomic TheoryNaqash RasheedNo ratings yet
- Janahi 12 Unit1 (Solid State)Document41 pagesJanahi 12 Unit1 (Solid State)ilias1973No ratings yet
- Thermo Vs KineticDocument6 pagesThermo Vs KineticPrathamesh DashNo ratings yet
- DegreeDocument4 pagesDegreePartha SenguptaNo ratings yet
- Bond EnthalpyDocument10 pagesBond EnthalpyPartha SenguptaNo ratings yet
- The First World War A Brief SummaryDocument4 pagesThe First World War A Brief SummaryPartha Sengupta100% (1)
- Trans EffectDocument6 pagesTrans EffectPrakash PhilipNo ratings yet
- Lande G FactorDocument9 pagesLande G FactorPartha SenguptaNo ratings yet
- Electron Spin ResonanceDocument49 pagesElectron Spin ResonancePartha SenguptaNo ratings yet
- Radical CationDocument65 pagesRadical CationPartha SenguptaNo ratings yet
- Born Haber CycleDocument16 pagesBorn Haber CyclePartha SenguptaNo ratings yet
- Ligand Field TheoryDocument35 pagesLigand Field TheoryPartha SenguptaNo ratings yet