Professional Documents
Culture Documents
Lesson 25: You Light Up My Life Classifying Substances.: Testing and Sorting Substances
Lesson 25: You Light Up My Life Classifying Substances.: Testing and Sorting Substances
Uploaded by
KaraLyn HatcherCopyright:
Available Formats
You might also like
- Larkin Lab ReportDocument3 pagesLarkin Lab ReportChristopherAguilar33% (3)
- Science Curriculum by Aaron KellerDocument7 pagesScience Curriculum by Aaron KellerResta THawNo ratings yet
- Chapter I - ElectrolysisDocument41 pagesChapter I - ElectrolysisRodella ChowdhuryNo ratings yet
- Electrolyte and NonDocument7 pagesElectrolyte and NonSuwahono, M.PdNo ratings yet
- Ionic vs. Covalent Bonding Lab InvestigationDocument4 pagesIonic vs. Covalent Bonding Lab InvestigationAngelicaNo ratings yet
- Electrochemistry: Electrolyte Vs Non-Electrolyte Conductor Vs Electrolyte Electrolysis Electrolytic CellDocument26 pagesElectrochemistry: Electrolyte Vs Non-Electrolyte Conductor Vs Electrolyte Electrolysis Electrolytic CellIzzati Zakirah Mohd GhazaliNo ratings yet
- ELECTROLYTES Mechanics AssignmentDocument4 pagesELECTROLYTES Mechanics Assignmentbighnesh.2006.singhNo ratings yet
- Chemistry (Chemical Energy) PDFDocument3 pagesChemistry (Chemical Energy) PDFMohammad RussellNo ratings yet
- LBC Alchemy SE Lesson26Document5 pagesLBC Alchemy SE Lesson26Sean MartinsonNo ratings yet
- Chemical Effects NotesDocument3 pagesChemical Effects NotessabreenaapNo ratings yet
- Electrochemistry: AY20/21 S1Q1 - M5Document15 pagesElectrochemistry: AY20/21 S1Q1 - M5Menaga A/P IlangkovanNo ratings yet
- Chemistry Lab PresentationDocument5 pagesChemistry Lab PresentationIhfaz NoorNo ratings yet
- Properties of Ionic and Covalent Compounds LabDocument1 pageProperties of Ionic and Covalent Compounds LabKevonSingh1No ratings yet
- 8 - Chemical Effects of CurrentDocument4 pages8 - Chemical Effects of CurrentÁbìńàv GNo ratings yet
- N 10 02 Chemical BondingDocument7 pagesN 10 02 Chemical BondingShivNo ratings yet
- ElectrochemistryDocument8 pagesElectrochemistryTeandraNo ratings yet
- Study Material - Chemical - Effect - of - Electric Current - classVIIIDocument15 pagesStudy Material - Chemical - Effect - of - Electric Current - classVIIIRajarshiNo ratings yet
- Electrolysis 2022-23Document18 pagesElectrolysis 2022-23Yasha RizviNo ratings yet
- Inquiry Into Bonding Lab - Intro TestDocument5 pagesInquiry Into Bonding Lab - Intro Testapi-491531529No ratings yet
- Meaning of ElectrolyteDocument12 pagesMeaning of ElectrolyteSuzilfarinda SamikNo ratings yet
- CSEC Chemistry - Structure and BondingDocument10 pagesCSEC Chemistry - Structure and BondingCornflakes ToastedNo ratings yet
- Chemical EffectDocument12 pagesChemical EffectMohan LalNo ratings yet
- Matthew McClain Lab Report Period 4Document4 pagesMatthew McClain Lab Report Period 4mmcclain2014No ratings yet
- 8 Thhourlab 9 BakerbrunatikallalkaneoberlanderDocument5 pages8 Thhourlab 9 Bakerbrunatikallalkaneoberlanderapi-297169088No ratings yet
- Electrochemistry and ElectrolysisDocument42 pagesElectrochemistry and ElectrolysisAnthonya KnightNo ratings yet
- L 14 Chemical Effects of Electric CurrentDocument31 pagesL 14 Chemical Effects of Electric CurrentSARANYA RAWATNo ratings yet
- 02 CHEM X ICSE SUMMARY Chemical BondingDocument9 pages02 CHEM X ICSE SUMMARY Chemical BondingNatasha DalalNo ratings yet
- Experiment 5 Dissimilarity Between Ionic and Covalent CompoundsDocument5 pagesExperiment 5 Dissimilarity Between Ionic and Covalent CompoundsNurasyilah YakubNo ratings yet
- Chemical Effects of Electric Current: AnswerDocument7 pagesChemical Effects of Electric Current: AnswerTvisha SolankiNo ratings yet
- Chemical Effects of Electric CurrentDocument9 pagesChemical Effects of Electric CurrentPrabal SinghNo ratings yet
- Christian Lara Lab ReportDocument3 pagesChristian Lara Lab ReportLeslieNo ratings yet
- Ionic vs. Covalent Bonding Lab Investigation: HypothesesDocument3 pagesIonic vs. Covalent Bonding Lab Investigation: HypothesesLeslieNo ratings yet
- C8 Chemical EffectDocument10 pagesC8 Chemical EffectorukathaisollungakalaijaiNo ratings yet
- Chem 503 - Activity 1Document5 pagesChem 503 - Activity 1Aries Jay ReyesNo ratings yet
- Chemical BondingDocument87 pagesChemical BondingMichael Dela Torre Diaz Jr.No ratings yet
- Assignment 1 Atomic StructureDocument9 pagesAssignment 1 Atomic StructureAnonymousNo ratings yet
- Science Notes Chapter 14 Chemical Effects of Electric Current (Class 8)Document10 pagesScience Notes Chapter 14 Chemical Effects of Electric Current (Class 8)Sifat MongaNo ratings yet
- Chemical Effets of El NVS TeachersDocument12 pagesChemical Effets of El NVS Teachersaashna shirbhateNo ratings yet
- Chemical Effects of Electric Current-Key NotesDocument6 pagesChemical Effects of Electric Current-Key Notestejasgoel2910No ratings yet
- Revision Notes Class 8 Science Chapter 14 - Chemical Effects of Electric CurrentDocument2 pagesRevision Notes Class 8 Science Chapter 14 - Chemical Effects of Electric CurrentArpit SharmaNo ratings yet
- g9 PPT 2nd QuarterDocument52 pagesg9 PPT 2nd Quarterjhaezhydomafergarcia2020No ratings yet
- Quickcard Class-VIII (Science) Chapter-14 Chemical Effects of Electric CurrentDocument5 pagesQuickcard Class-VIII (Science) Chapter-14 Chemical Effects of Electric CurrentTridha DuttaNo ratings yet
- Practica 1 ElectroDocument14 pagesPractica 1 ElectroAnna KoronaNo ratings yet
- Structure and Bonding (1)Document37 pagesStructure and Bonding (1)alexia.farrellNo ratings yet
- Bonding and Properties of SubstancesDocument3 pagesBonding and Properties of Substancesdan964No ratings yet
- Chap 11: Electrolysis: ProcessDocument2 pagesChap 11: Electrolysis: ProcessAbdur RehmanNo ratings yet
- Bonding in SolidsDocument31 pagesBonding in Solidspraveen4u_happyNo ratings yet
- Chemical Bonding IIDocument7 pagesChemical Bonding IIdanielmahsaNo ratings yet
- Bonding Lab RportDocument3 pagesBonding Lab RportMarlynNo ratings yet
- Electric CurrentDocument12 pagesElectric Currentstory manNo ratings yet
- Identifying Substances Based On Bonding PropertiesDocument2 pagesIdentifying Substances Based On Bonding Propertiesapi-239642851No ratings yet
- Bonding in SolidsDocument31 pagesBonding in SolidsReddyvari VenugopalNo ratings yet
- Structure of MoleculesDocument11 pagesStructure of MoleculesalxsxxdNo ratings yet
- The Name's Bonds, Breaking BondsDocument6 pagesThe Name's Bonds, Breaking Bondsapi-348321624No ratings yet
- Electrolysis IntroductionDocument6 pagesElectrolysis IntroductionAngel MulyadiNo ratings yet
- Lecture 5: Bonding Models: Ionic BondsDocument4 pagesLecture 5: Bonding Models: Ionic BondsShreyas KamathNo ratings yet
- L7 - Ionic Compounds PropertiesDocument19 pagesL7 - Ionic Compounds PropertiesKashifNo ratings yet
- Practice Makes Perfect in Chemistry: Chemical BondingFrom EverandPractice Makes Perfect in Chemistry: Chemical BondingRating: 5 out of 5 stars5/5 (3)
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)
- HyperthyroidismDocument11 pagesHyperthyroidismKaraLyn HatcherNo ratings yet
- Study Guide - Ch. 28 The Crisis of The Imperial Order 1900-1929Document2 pagesStudy Guide - Ch. 28 The Crisis of The Imperial Order 1900-1929KaraLyn HatcherNo ratings yet
- Psychological Disorders BookletDocument16 pagesPsychological Disorders BookletKaraLyn Hatcher100% (1)
- Chapter 27 NotesDocument62 pagesChapter 27 NotesKaraLyn HatcherNo ratings yet
- Unit 3 Practice ExamDocument3 pagesUnit 3 Practice ExamKaraLyn HatcherNo ratings yet
- Ap Human Geography Migration ProjectDocument3 pagesAp Human Geography Migration ProjectKaraLyn HatcherNo ratings yet
- LatinDocument1 pageLatinKaraLyn HatcherNo ratings yet
Lesson 25: You Light Up My Life Classifying Substances.: Testing and Sorting Substances
Lesson 25: You Light Up My Life Classifying Substances.: Testing and Sorting Substances
Uploaded by
KaraLyn HatcherOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lesson 25: You Light Up My Life Classifying Substances.: Testing and Sorting Substances
Lesson 25: You Light Up My Life Classifying Substances.: Testing and Sorting Substances
Uploaded by
KaraLyn HatcherCopyright:
Available Formats
Carolyn Hatcher Period 4B
Some substances dissolve in water and others do not. So, dissolving is one property of matter that you can use to sort substances into general groups. A substance that dissolves in water is soluble in water. A substance that does not dissolve is insoluble in water.
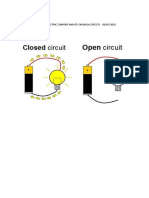
The property of conductivity has to do with whether a substance will conduct electricity. Electrical conductivity requires the movement of ions or electrons.
Dissolving and Conductivity
Lesson 25: You light up my life; classifying substances.
The substances that are soluble in water and conduct electricity are made up of a metal element and one or more nonmetal elements. These are called ionic compounds.
Electrical conductivity can be tested by setting up a simple electrical circuit. Wires connect the terminals of a battery to a light bulb. When the circuit is complete, and an electrical current is flowing, the bulb will light up. If the flow of current is interrupted by the presence of a substance that does not conduct electricity, the light bulb will not light up.
Testing and Sorting Substances
The substances that are insoluble in water but do conduct electricity are made of metallic elements.
The substances that are soluble in water but do not conduct electricity are often made up of carbon, hydrogen, and oxygen atoms joined together. These are all nonmetal elements.
The substances that are insoluble in water and do not conduct electricity are made up of nonmetal atoms.
Ionic compounds conduct electricity only when they are dissolved in water. Dry ionic solids do not conduct electricity.
You might also like
- Larkin Lab ReportDocument3 pagesLarkin Lab ReportChristopherAguilar33% (3)
- Science Curriculum by Aaron KellerDocument7 pagesScience Curriculum by Aaron KellerResta THawNo ratings yet
- Chapter I - ElectrolysisDocument41 pagesChapter I - ElectrolysisRodella ChowdhuryNo ratings yet
- Electrolyte and NonDocument7 pagesElectrolyte and NonSuwahono, M.PdNo ratings yet
- Ionic vs. Covalent Bonding Lab InvestigationDocument4 pagesIonic vs. Covalent Bonding Lab InvestigationAngelicaNo ratings yet
- Electrochemistry: Electrolyte Vs Non-Electrolyte Conductor Vs Electrolyte Electrolysis Electrolytic CellDocument26 pagesElectrochemistry: Electrolyte Vs Non-Electrolyte Conductor Vs Electrolyte Electrolysis Electrolytic CellIzzati Zakirah Mohd GhazaliNo ratings yet
- ELECTROLYTES Mechanics AssignmentDocument4 pagesELECTROLYTES Mechanics Assignmentbighnesh.2006.singhNo ratings yet
- Chemistry (Chemical Energy) PDFDocument3 pagesChemistry (Chemical Energy) PDFMohammad RussellNo ratings yet
- LBC Alchemy SE Lesson26Document5 pagesLBC Alchemy SE Lesson26Sean MartinsonNo ratings yet
- Chemical Effects NotesDocument3 pagesChemical Effects NotessabreenaapNo ratings yet
- Electrochemistry: AY20/21 S1Q1 - M5Document15 pagesElectrochemistry: AY20/21 S1Q1 - M5Menaga A/P IlangkovanNo ratings yet
- Chemistry Lab PresentationDocument5 pagesChemistry Lab PresentationIhfaz NoorNo ratings yet
- Properties of Ionic and Covalent Compounds LabDocument1 pageProperties of Ionic and Covalent Compounds LabKevonSingh1No ratings yet
- 8 - Chemical Effects of CurrentDocument4 pages8 - Chemical Effects of CurrentÁbìńàv GNo ratings yet
- N 10 02 Chemical BondingDocument7 pagesN 10 02 Chemical BondingShivNo ratings yet
- ElectrochemistryDocument8 pagesElectrochemistryTeandraNo ratings yet
- Study Material - Chemical - Effect - of - Electric Current - classVIIIDocument15 pagesStudy Material - Chemical - Effect - of - Electric Current - classVIIIRajarshiNo ratings yet
- Electrolysis 2022-23Document18 pagesElectrolysis 2022-23Yasha RizviNo ratings yet
- Inquiry Into Bonding Lab - Intro TestDocument5 pagesInquiry Into Bonding Lab - Intro Testapi-491531529No ratings yet
- Meaning of ElectrolyteDocument12 pagesMeaning of ElectrolyteSuzilfarinda SamikNo ratings yet
- CSEC Chemistry - Structure and BondingDocument10 pagesCSEC Chemistry - Structure and BondingCornflakes ToastedNo ratings yet
- Chemical EffectDocument12 pagesChemical EffectMohan LalNo ratings yet
- Matthew McClain Lab Report Period 4Document4 pagesMatthew McClain Lab Report Period 4mmcclain2014No ratings yet
- 8 Thhourlab 9 BakerbrunatikallalkaneoberlanderDocument5 pages8 Thhourlab 9 Bakerbrunatikallalkaneoberlanderapi-297169088No ratings yet
- Electrochemistry and ElectrolysisDocument42 pagesElectrochemistry and ElectrolysisAnthonya KnightNo ratings yet
- L 14 Chemical Effects of Electric CurrentDocument31 pagesL 14 Chemical Effects of Electric CurrentSARANYA RAWATNo ratings yet
- 02 CHEM X ICSE SUMMARY Chemical BondingDocument9 pages02 CHEM X ICSE SUMMARY Chemical BondingNatasha DalalNo ratings yet
- Experiment 5 Dissimilarity Between Ionic and Covalent CompoundsDocument5 pagesExperiment 5 Dissimilarity Between Ionic and Covalent CompoundsNurasyilah YakubNo ratings yet
- Chemical Effects of Electric Current: AnswerDocument7 pagesChemical Effects of Electric Current: AnswerTvisha SolankiNo ratings yet
- Chemical Effects of Electric CurrentDocument9 pagesChemical Effects of Electric CurrentPrabal SinghNo ratings yet
- Christian Lara Lab ReportDocument3 pagesChristian Lara Lab ReportLeslieNo ratings yet
- Ionic vs. Covalent Bonding Lab Investigation: HypothesesDocument3 pagesIonic vs. Covalent Bonding Lab Investigation: HypothesesLeslieNo ratings yet
- C8 Chemical EffectDocument10 pagesC8 Chemical EffectorukathaisollungakalaijaiNo ratings yet
- Chem 503 - Activity 1Document5 pagesChem 503 - Activity 1Aries Jay ReyesNo ratings yet
- Chemical BondingDocument87 pagesChemical BondingMichael Dela Torre Diaz Jr.No ratings yet
- Assignment 1 Atomic StructureDocument9 pagesAssignment 1 Atomic StructureAnonymousNo ratings yet
- Science Notes Chapter 14 Chemical Effects of Electric Current (Class 8)Document10 pagesScience Notes Chapter 14 Chemical Effects of Electric Current (Class 8)Sifat MongaNo ratings yet
- Chemical Effets of El NVS TeachersDocument12 pagesChemical Effets of El NVS Teachersaashna shirbhateNo ratings yet
- Chemical Effects of Electric Current-Key NotesDocument6 pagesChemical Effects of Electric Current-Key Notestejasgoel2910No ratings yet
- Revision Notes Class 8 Science Chapter 14 - Chemical Effects of Electric CurrentDocument2 pagesRevision Notes Class 8 Science Chapter 14 - Chemical Effects of Electric CurrentArpit SharmaNo ratings yet
- g9 PPT 2nd QuarterDocument52 pagesg9 PPT 2nd Quarterjhaezhydomafergarcia2020No ratings yet
- Quickcard Class-VIII (Science) Chapter-14 Chemical Effects of Electric CurrentDocument5 pagesQuickcard Class-VIII (Science) Chapter-14 Chemical Effects of Electric CurrentTridha DuttaNo ratings yet
- Practica 1 ElectroDocument14 pagesPractica 1 ElectroAnna KoronaNo ratings yet
- Structure and Bonding (1)Document37 pagesStructure and Bonding (1)alexia.farrellNo ratings yet
- Bonding and Properties of SubstancesDocument3 pagesBonding and Properties of Substancesdan964No ratings yet
- Chap 11: Electrolysis: ProcessDocument2 pagesChap 11: Electrolysis: ProcessAbdur RehmanNo ratings yet
- Bonding in SolidsDocument31 pagesBonding in Solidspraveen4u_happyNo ratings yet
- Chemical Bonding IIDocument7 pagesChemical Bonding IIdanielmahsaNo ratings yet
- Bonding Lab RportDocument3 pagesBonding Lab RportMarlynNo ratings yet
- Electric CurrentDocument12 pagesElectric Currentstory manNo ratings yet
- Identifying Substances Based On Bonding PropertiesDocument2 pagesIdentifying Substances Based On Bonding Propertiesapi-239642851No ratings yet
- Bonding in SolidsDocument31 pagesBonding in SolidsReddyvari VenugopalNo ratings yet
- Structure of MoleculesDocument11 pagesStructure of MoleculesalxsxxdNo ratings yet
- The Name's Bonds, Breaking BondsDocument6 pagesThe Name's Bonds, Breaking Bondsapi-348321624No ratings yet
- Electrolysis IntroductionDocument6 pagesElectrolysis IntroductionAngel MulyadiNo ratings yet
- Lecture 5: Bonding Models: Ionic BondsDocument4 pagesLecture 5: Bonding Models: Ionic BondsShreyas KamathNo ratings yet
- L7 - Ionic Compounds PropertiesDocument19 pagesL7 - Ionic Compounds PropertiesKashifNo ratings yet
- Practice Makes Perfect in Chemistry: Chemical BondingFrom EverandPractice Makes Perfect in Chemistry: Chemical BondingRating: 5 out of 5 stars5/5 (3)
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)
- HyperthyroidismDocument11 pagesHyperthyroidismKaraLyn HatcherNo ratings yet
- Study Guide - Ch. 28 The Crisis of The Imperial Order 1900-1929Document2 pagesStudy Guide - Ch. 28 The Crisis of The Imperial Order 1900-1929KaraLyn HatcherNo ratings yet
- Psychological Disorders BookletDocument16 pagesPsychological Disorders BookletKaraLyn Hatcher100% (1)
- Chapter 27 NotesDocument62 pagesChapter 27 NotesKaraLyn HatcherNo ratings yet
- Unit 3 Practice ExamDocument3 pagesUnit 3 Practice ExamKaraLyn HatcherNo ratings yet
- Ap Human Geography Migration ProjectDocument3 pagesAp Human Geography Migration ProjectKaraLyn HatcherNo ratings yet
- LatinDocument1 pageLatinKaraLyn HatcherNo ratings yet