Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
83 viewsEx 9
Ex 9
Uploaded by
3a2dThis document summarizes the results of two chemical reactions:
1) The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) in a 1:1 molar ratio, producing sodium chloride (NaCl), water (H2O), and a change in temperature.
2) The reaction between sulfuric acid (H2SO4) and sodium hydroxide (NaOH) in a 1:2 molar ratio, producing sodium sulfate (Na2SO4), water (H2O), and a greater change in temperature than the first reaction due to H2SO4 being a stronger acid.
3) The differences between the temperature change graphs of
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You might also like
- 2010 CC 3.6 Engine Schematic R36Document19 pages2010 CC 3.6 Engine Schematic R36Dungani AllanNo ratings yet
- Nice3000new Diagram PDFDocument33 pagesNice3000new Diagram PDFAsdrubal Fredy Gutierrez100% (7)
- Nov 2022 H2 Chemistry 9729 Paper 4 Suggested SolutionDocument20 pagesNov 2022 H2 Chemistry 9729 Paper 4 Suggested Solutionzavairling05No ratings yet
- Lab Report Experiment 1 - Rate of Reaction - 2021Document4 pagesLab Report Experiment 1 - Rate of Reaction - 2021Ye Woon LimNo ratings yet
- Title: Enthalpy Objective: 1. To Determine The Enthalpy of Neutralization of Strong Acid and Strong BaseDocument10 pagesTitle: Enthalpy Objective: 1. To Determine The Enthalpy of Neutralization of Strong Acid and Strong BaseAnonymous eGc6IFJc8GNo ratings yet
- CAPE Chemistry U2 Lab - Thermometric Titration (SAMPLE)Document5 pagesCAPE Chemistry U2 Lab - Thermometric Titration (SAMPLE)Haxara SimsNo ratings yet
- 5.1.1 Exercise 1 - CONCENTRATION-TIME GRAPHSDocument2 pages5.1.1 Exercise 1 - CONCENTRATION-TIME GRAPHSIqbal Hossain ZahidNo ratings yet
- Determination of Heat of SolutionDocument6 pagesDetermination of Heat of SolutionRafid Jawad100% (2)
- ch03 SM Chemistry2eDocument36 pagesch03 SM Chemistry2eLLL0% (1)
- Heat & Determining Enthalpy Change (Lab Assessment) Part I & Part 2Document8 pagesHeat & Determining Enthalpy Change (Lab Assessment) Part I & Part 2Mark Riley81% (16)
- Analytical Chemistry Lab CHDocument22 pagesAnalytical Chemistry Lab CHm.shehroz8898No ratings yet
- Science Rate of Reaction Vinegar Baking SodaDocument4 pagesScience Rate of Reaction Vinegar Baking Sodadbwhwd qwdwNo ratings yet
- Lab Report Experiment 1 CHM524Document16 pagesLab Report Experiment 1 CHM524Hazwan Hamim67% (3)
- Practicum 6Document7 pagesPracticum 6Farras Reyhan HidayatullahNo ratings yet
- Problems StoichiometryDocument2 pagesProblems StoichiometryAida Saefa FitriarieswaNo ratings yet
- CHEMISTRY Lab Report - THER 368Document19 pagesCHEMISTRY Lab Report - THER 368kaleb16_2No ratings yet
- Experiment 1 Lab ReportDocument7 pagesExperiment 1 Lab ReportChaapi KimNo ratings yet
- DataDocument3 pagesDataClaudia SalazarNo ratings yet
- CAPE Chemistry Lab 11Document6 pagesCAPE Chemistry Lab 11Aiden BabwahNo ratings yet
- Kinetics (Gjjkkkgty)Document5 pagesKinetics (Gjjkkkgty)Chrysler Kane DepnagNo ratings yet
- Thermometeric TitrationDocument2 pagesThermometeric Titrationabriannathomas123No ratings yet
- An Chem II Practical 1Document8 pagesAn Chem II Practical 1Nhlakanipho VilakaziNo ratings yet
- Heat Release in A Neutralization Reaction and Acid Strength - CleanedDocument8 pagesHeat Release in A Neutralization Reaction and Acid Strength - CleanedIbrahim AliNo ratings yet
- Analisis Pembahasan Laju ReaksiDocument11 pagesAnalisis Pembahasan Laju ReaksiLia Yuli KusumaNo ratings yet
- Kinetics 1Document3 pagesKinetics 1JuarezNo ratings yet
- Model Exam G12 ChemistryDocument12 pagesModel Exam G12 ChemistryNebilahNo ratings yet
- Final AssessmentDocument10 pagesFinal AssessmentDiogo Tavares de OliveiraNo ratings yet
- Chemistry: Year 12 Assessment Block Semester 1Document23 pagesChemistry: Year 12 Assessment Block Semester 1nichollsl24No ratings yet
- Experiment 2Document7 pagesExperiment 2EDWIN SIMBARASHE MASUNUNGURENo ratings yet
- Topic 11 Exercise 2 - Concentration-Time GraphsDocument2 pagesTopic 11 Exercise 2 - Concentration-Time GraphshusainNo ratings yet
- Stoic PracticeDocument1 pageStoic PracticeJannette JaneNo ratings yet
- PTG Chapter 3 Asal ChemistryDocument15 pagesPTG Chapter 3 Asal ChemistryKai GeeNo ratings yet
- Chemistry Trial HSC 2023 (WITH LINES)Document28 pagesChemistry Trial HSC 2023 (WITH LINES)bianhua006No ratings yet
- Reaction KineticsDocument97 pagesReaction KineticsMuhammad Khuzaifah AzliNo ratings yet
- Changing The Rate of A Reaction (Part I)Document2 pagesChanging The Rate of A Reaction (Part I)Shahid Ur RehmanNo ratings yet
- Unit 4 Review Reaction Rates Answers To ReviewDocument8 pagesUnit 4 Review Reaction Rates Answers To ReviewANGELYN SANTOSNo ratings yet
- A00830666 - Lab Report - BatchDocument13 pagesA00830666 - Lab Report - BatchJocelyn garcia gonzalezNo ratings yet
- Determining The Order of A Chemical Reaction LabDocument8 pagesDetermining The Order of A Chemical Reaction LabJonathan_Khan7100% (1)
- Heat of NeutralisationDocument3 pagesHeat of NeutralisationCynthia RoneyNo ratings yet
- Chemical Exp 8Document10 pagesChemical Exp 8门门No ratings yet
- T2 - E5-Phase Transfer CatalystDocument14 pagesT2 - E5-Phase Transfer Catalystabhisheknemade9765No ratings yet
- CHE1700Document9 pagesCHE1700rachzammit2003No ratings yet
- Labsheet No.1: Kashif Husaain Sp-18-Bschem-004Document6 pagesLabsheet No.1: Kashif Husaain Sp-18-Bschem-004Muyammad Ahsan abbasNo ratings yet
- Year 10 Semester One Revision Sheet 6 ANSWERSDocument4 pagesYear 10 Semester One Revision Sheet 6 ANSWERSsports.kingdomNo ratings yet
- Purpose: Lab Work 1. Chemical Kinetics. Chemical EquilibriumDocument5 pagesPurpose: Lab Work 1. Chemical Kinetics. Chemical EquilibriumFritz ManyauNo ratings yet
- Experimental Report PDFDocument8 pagesExperimental Report PDFChrisNo ratings yet
- Ex.3-Heat of NeutralizationDocument10 pagesEx.3-Heat of Neutralizationalia2003skNo ratings yet
- 2012fall 113 FinalDocument15 pages2012fall 113 FinalSrynnENo ratings yet
- Data Sheet For Exp 1Document2 pagesData Sheet For Exp 1DanaNo ratings yet
- Benzoic - Acid ExperimentDocument7 pagesBenzoic - Acid ExperimentShivani SinghNo ratings yet
- Tutorial-Manual CH1002Document18 pagesTutorial-Manual CH1002Gift Chulu100% (2)
- Topic 6 16 MC PracticeDocument25 pagesTopic 6 16 MC PracticeDharmesh Ramnarayan YadavNo ratings yet
- 61-Rate of Reaction QP o Level - Cie - ChemistryDocument21 pages61-Rate of Reaction QP o Level - Cie - ChemistryZaid BabarNo ratings yet
- Appendix V L. Determination of PH Values - British PharmacopoeiaDocument3 pagesAppendix V L. Determination of PH Values - British PharmacopoeiaAbd El-Rahman SayedNo ratings yet
- 2020 - Equilibrium Practice ProblemsDocument5 pages2020 - Equilibrium Practice ProblemsAMOS SODJAHINNo ratings yet
- 2013 Usnco Local SolutionsDocument10 pages2013 Usnco Local SolutionsPelatihan KimiaNo ratings yet
- Practical LabDocument7 pagesPractical Labdaudiali2002No ratings yet
- Rate of ReactionDocument12 pagesRate of Reactionsurvival vlogsNo ratings yet
- Journal of Colligative PropertiesDocument9 pagesJournal of Colligative PropertiesMuhammad Baihaqi100% (1)
- Density of LiquidsDocument17 pagesDensity of LiquidsISAYA KICHELENo ratings yet
- Ap Unit7 WorksheetDocument4 pagesAp Unit7 Worksheetburcak gecNo ratings yet
- Ex 6Document2 pagesEx 63a2dNo ratings yet
- Ex 7Document3 pagesEx 73a2dNo ratings yet
- Ex 5Document2 pagesEx 53a2dNo ratings yet
- Ex 3Document2 pagesEx 33a2dNo ratings yet
- Ex 4Document2 pagesEx 43a2dNo ratings yet
- Experiment 2: "Determination of Boiling and Melting Points of Chemical Compounds" I. Observations and ResultsDocument2 pagesExperiment 2: "Determination of Boiling and Melting Points of Chemical Compounds" I. Observations and Results3a2dNo ratings yet
- Objectives:: AC Steady State PowerDocument5 pagesObjectives:: AC Steady State Power3a2dNo ratings yet
- Ex 1Document3 pagesEx 13a2d0% (1)
- Experiement 6Document3 pagesExperiement 63a2dNo ratings yet
- Experiement 5Document9 pagesExperiement 53a2dNo ratings yet
- Exp 9Document6 pagesExp 93a2dNo ratings yet
- Objective:: Electric LabDocument5 pagesObjective:: Electric Lab3a2dNo ratings yet
- Objective:: Electric LabDocument3 pagesObjective:: Electric Lab3a2dNo ratings yet
- مقدمةDocument1 pageمقدمة3a2dNo ratings yet
- Electric Lab .: The Basic Resistance MeasurementsDocument6 pagesElectric Lab .: The Basic Resistance Measurements3a2dNo ratings yet
- Objective:: Electric LabDocument8 pagesObjective:: Electric Lab3a2dNo ratings yet
- CyclamenDocument9 pagesCyclamenLAUM1No ratings yet
- Ideas and Methods of Turbomachinery Aerodynamics A Historical ViewDocument12 pagesIdeas and Methods of Turbomachinery Aerodynamics A Historical ViewLos MagandosNo ratings yet
- SOCIOLOGICAL FOUNDATION ReportDocument14 pagesSOCIOLOGICAL FOUNDATION Reportᜃᜒᜈ᜔ᜎᜒ ᜇᜒᜋᜈ᜔ᜇᜒᜋᜈ᜔No ratings yet
- Amity: UniversityDocument10 pagesAmity: UniversityVinayak MangotraNo ratings yet
- Unit-1 Introduction To Management and OrganizationsDocument73 pagesUnit-1 Introduction To Management and OrganizationsGnanesh GNo ratings yet
- Metriso 5000ADocument14 pagesMetriso 5000Ahellfire22000No ratings yet
- AIT Unit1 InternetDocument44 pagesAIT Unit1 InternetNaina_Dwivedi_6514No ratings yet
- Fundmentals Energy ProcessDocument11 pagesFundmentals Energy ProcessepjxNo ratings yet
- Postgraduate Prospectus For 2014 EntryDocument296 pagesPostgraduate Prospectus For 2014 EntryDaniel LieNo ratings yet
- 6chefs Plate - Fresh Ingredients & Delicious Recipes Delivered To Your DoorDocument6 pages6chefs Plate - Fresh Ingredients & Delicious Recipes Delivered To Your DoorEmmaNo ratings yet
- Why Electrical EngineeringDocument31 pagesWhy Electrical Engineeringsyed42210No ratings yet
- Eastern Europe ManufacturingDocument14 pagesEastern Europe ManufacturingNB Thushara HarithasNo ratings yet
- PDF Data Science and Machine Learning Mathematical and Statistical Methods Chapman Hall CRC Machine Learning Pattern Recognition 1St Edition Dirk P Kroese Ebook Full ChapterDocument54 pagesPDF Data Science and Machine Learning Mathematical and Statistical Methods Chapman Hall CRC Machine Learning Pattern Recognition 1St Edition Dirk P Kroese Ebook Full Chaptercatherine.cottingham887100% (5)
- SKYPAD Pocket Firmware Instructions PDFDocument3 pagesSKYPAD Pocket Firmware Instructions PDFAbelito FloresNo ratings yet
- Engels As Interpreter of Marx's EconomicsDocument37 pagesEngels As Interpreter of Marx's EconomicsJuan HermidasNo ratings yet
- Answer Keys For Problem Set 3: MIT 14.04 Intermediate Microeconomic Theory Fall 2003Document4 pagesAnswer Keys For Problem Set 3: MIT 14.04 Intermediate Microeconomic Theory Fall 2003Flavio Andrés Eichin CamposNo ratings yet
- ASUS RT-AC58U ManualDocument122 pagesASUS RT-AC58U ManualSeungpyo HongNo ratings yet
- Design Simulation and Realization of Solar Battery Charge Controller Using Arduino UnoDocument5 pagesDesign Simulation and Realization of Solar Battery Charge Controller Using Arduino UnoRushiraj DesaiNo ratings yet
- Department of Education: A. Background Information For Learners B. Most Essential Learning CompetencyDocument9 pagesDepartment of Education: A. Background Information For Learners B. Most Essential Learning CompetencyCamille CaigaNo ratings yet
- Infectious Diseases 2022 Virtual Abstract BookDocument53 pagesInfectious Diseases 2022 Virtual Abstract BookSriNo ratings yet
- V. Evaporator Design 1. Evaporator and Water Treatment EvaporatorDocument3 pagesV. Evaporator Design 1. Evaporator and Water Treatment EvaporatorEngelbert AntodNo ratings yet
- MCDonald's StrategyDocument3 pagesMCDonald's StrategyShaukat HasanNo ratings yet
- Sarah Ribeiro ResumeDocument1 pageSarah Ribeiro ResumeSarah RibeiroNo ratings yet
- MTTRAPD Huaweii2000 cl2Document3,295 pagesMTTRAPD Huaweii2000 cl2Muhammad AdnanNo ratings yet
- Assignment 2 Lesson Plan AnalysisDocument14 pagesAssignment 2 Lesson Plan Analysisapi-373008418No ratings yet
- His 418 SinenkosiDocument7 pagesHis 418 Sinenkosisiphiwo dlaminiNo ratings yet
- Fiqh of MarriageDocument30 pagesFiqh of MarriageNawaid ZamanNo ratings yet
- Guru 1 - 5 Whats NewDocument19 pagesGuru 1 - 5 Whats NewLuca RosacutaNo ratings yet
Ex 9
Ex 9
Uploaded by
3a2d0 ratings0% found this document useful (0 votes)
83 views2 pagesThis document summarizes the results of two chemical reactions:
1) The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) in a 1:1 molar ratio, producing sodium chloride (NaCl), water (H2O), and a change in temperature.
2) The reaction between sulfuric acid (H2SO4) and sodium hydroxide (NaOH) in a 1:2 molar ratio, producing sodium sulfate (Na2SO4), water (H2O), and a greater change in temperature than the first reaction due to H2SO4 being a stronger acid.
3) The differences between the temperature change graphs of
Original Description:
chemical laboratory
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document summarizes the results of two chemical reactions:
1) The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) in a 1:1 molar ratio, producing sodium chloride (NaCl), water (H2O), and a change in temperature.
2) The reaction between sulfuric acid (H2SO4) and sodium hydroxide (NaOH) in a 1:2 molar ratio, producing sodium sulfate (Na2SO4), water (H2O), and a greater change in temperature than the first reaction due to H2SO4 being a stronger acid.
3) The differences between the temperature change graphs of
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
0 ratings0% found this document useful (0 votes)
83 views2 pagesEx 9
Ex 9
Uploaded by
3a2dThis document summarizes the results of two chemical reactions:
1) The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) in a 1:1 molar ratio, producing sodium chloride (NaCl), water (H2O), and a change in temperature.
2) The reaction between sulfuric acid (H2SO4) and sodium hydroxide (NaOH) in a 1:2 molar ratio, producing sodium sulfate (Na2SO4), water (H2O), and a greater change in temperature than the first reaction due to H2SO4 being a stronger acid.
3) The differences between the temperature change graphs of
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
You are on page 1of 2
بسم ال الرحمن الرحيم
Name: ayedzahran
Group:
Day:
Experiment 9
"Stoichiometry of two chemical reaction"
__________________________________________________________________
I. Observations and results:
A. Reaction between HCl and NaOH:
Table 1
ml 1 M HCl ml 1 M NaOH Temperature change
5 45 25-24=1 Cº
10 40 25-23.5=1.5 Cº
15 35 25.5-22.5=2 Cº
20 30 26-23=3 Cº
25 25 27-23=4 Cº
30 20 26-22.5=3.5 Cº
35 15 24-22=2 Cº
40 10 22.5-21.5=1 Cº
45 5 22-21.5=0.5 Cº
B. Reaction between H2SO4 and NaOH
Table 2
ml 1 M H2SO4 ml 1 M NaOH Temperature change
5 20 Cº 30.5-23=7.5
10 20 Cº 10.5=33.5-23
15 20 Cº 9.5=33-23.5
20 20 Cº 9=32-23
25 20 Cº 8=31-23
30 20 Cº 7.5=30.5-23
35 20 Cº 6.5=29.5-23
40 20 Cº 6=29-23
45 20 Cº 5.5=28-22.5
III. Answer the following questions:
1. Based on your results above, in what molar ratio do:
a. HCl and NaOH react: 1:1
b. H2SO4 and NaOH react: 1:2
2. Write balance equation for the reaction between:
a. HCl and NaOH:
HCl + NaOH NaCl + H2O + ΔH
b. H2SO4 and NaOH:
H2SO4 + 2NaOH Na2SO4 + 2H2O + ΔH
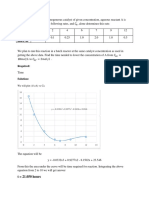
3. What is the difference between the forms of graph obtained in part A
and B of this experiment? Explain your answer.
The difference between part A and B:
A. The molar ratio in part A is 1:1, HCl:NaOH. and in B is 1:2
H2SO4:NaOH, we known that from the max change of heat, in
that’s point in A was when the volume of the reactions is the
same, and in B was when NaOH is 20 ml and H2SO4 is 10 ml.
B. The change of temperature in B mar than A, because H2SO4 is
stronger than HCl.
C. In A the curve start from 0 and end from 0, but in B start from
0 and goes to 0 after many times (when H2SO4 larger than
NaOH many times),because in A the two reactions is change
in volume so when the volume of any two reactions is 0 that’s
mean no reaction so no change in temperature, in B the
volume of NaOH is constant (20 ml) that’s mean the change
of temperature goes to 0 (≈ 0) when the volume of H2SO4
more and more than NaOH, because the large volume and low
heat.
Not: For experiment number, For calculation number, For the solution of
answers.
You might also like
- 2010 CC 3.6 Engine Schematic R36Document19 pages2010 CC 3.6 Engine Schematic R36Dungani AllanNo ratings yet
- Nice3000new Diagram PDFDocument33 pagesNice3000new Diagram PDFAsdrubal Fredy Gutierrez100% (7)
- Nov 2022 H2 Chemistry 9729 Paper 4 Suggested SolutionDocument20 pagesNov 2022 H2 Chemistry 9729 Paper 4 Suggested Solutionzavairling05No ratings yet
- Lab Report Experiment 1 - Rate of Reaction - 2021Document4 pagesLab Report Experiment 1 - Rate of Reaction - 2021Ye Woon LimNo ratings yet
- Title: Enthalpy Objective: 1. To Determine The Enthalpy of Neutralization of Strong Acid and Strong BaseDocument10 pagesTitle: Enthalpy Objective: 1. To Determine The Enthalpy of Neutralization of Strong Acid and Strong BaseAnonymous eGc6IFJc8GNo ratings yet
- CAPE Chemistry U2 Lab - Thermometric Titration (SAMPLE)Document5 pagesCAPE Chemistry U2 Lab - Thermometric Titration (SAMPLE)Haxara SimsNo ratings yet
- 5.1.1 Exercise 1 - CONCENTRATION-TIME GRAPHSDocument2 pages5.1.1 Exercise 1 - CONCENTRATION-TIME GRAPHSIqbal Hossain ZahidNo ratings yet
- Determination of Heat of SolutionDocument6 pagesDetermination of Heat of SolutionRafid Jawad100% (2)
- ch03 SM Chemistry2eDocument36 pagesch03 SM Chemistry2eLLL0% (1)
- Heat & Determining Enthalpy Change (Lab Assessment) Part I & Part 2Document8 pagesHeat & Determining Enthalpy Change (Lab Assessment) Part I & Part 2Mark Riley81% (16)
- Analytical Chemistry Lab CHDocument22 pagesAnalytical Chemistry Lab CHm.shehroz8898No ratings yet
- Science Rate of Reaction Vinegar Baking SodaDocument4 pagesScience Rate of Reaction Vinegar Baking Sodadbwhwd qwdwNo ratings yet
- Lab Report Experiment 1 CHM524Document16 pagesLab Report Experiment 1 CHM524Hazwan Hamim67% (3)
- Practicum 6Document7 pagesPracticum 6Farras Reyhan HidayatullahNo ratings yet
- Problems StoichiometryDocument2 pagesProblems StoichiometryAida Saefa FitriarieswaNo ratings yet
- CHEMISTRY Lab Report - THER 368Document19 pagesCHEMISTRY Lab Report - THER 368kaleb16_2No ratings yet
- Experiment 1 Lab ReportDocument7 pagesExperiment 1 Lab ReportChaapi KimNo ratings yet
- DataDocument3 pagesDataClaudia SalazarNo ratings yet
- CAPE Chemistry Lab 11Document6 pagesCAPE Chemistry Lab 11Aiden BabwahNo ratings yet
- Kinetics (Gjjkkkgty)Document5 pagesKinetics (Gjjkkkgty)Chrysler Kane DepnagNo ratings yet
- Thermometeric TitrationDocument2 pagesThermometeric Titrationabriannathomas123No ratings yet
- An Chem II Practical 1Document8 pagesAn Chem II Practical 1Nhlakanipho VilakaziNo ratings yet
- Heat Release in A Neutralization Reaction and Acid Strength - CleanedDocument8 pagesHeat Release in A Neutralization Reaction and Acid Strength - CleanedIbrahim AliNo ratings yet
- Analisis Pembahasan Laju ReaksiDocument11 pagesAnalisis Pembahasan Laju ReaksiLia Yuli KusumaNo ratings yet
- Kinetics 1Document3 pagesKinetics 1JuarezNo ratings yet
- Model Exam G12 ChemistryDocument12 pagesModel Exam G12 ChemistryNebilahNo ratings yet
- Final AssessmentDocument10 pagesFinal AssessmentDiogo Tavares de OliveiraNo ratings yet
- Chemistry: Year 12 Assessment Block Semester 1Document23 pagesChemistry: Year 12 Assessment Block Semester 1nichollsl24No ratings yet
- Experiment 2Document7 pagesExperiment 2EDWIN SIMBARASHE MASUNUNGURENo ratings yet
- Topic 11 Exercise 2 - Concentration-Time GraphsDocument2 pagesTopic 11 Exercise 2 - Concentration-Time GraphshusainNo ratings yet
- Stoic PracticeDocument1 pageStoic PracticeJannette JaneNo ratings yet
- PTG Chapter 3 Asal ChemistryDocument15 pagesPTG Chapter 3 Asal ChemistryKai GeeNo ratings yet
- Chemistry Trial HSC 2023 (WITH LINES)Document28 pagesChemistry Trial HSC 2023 (WITH LINES)bianhua006No ratings yet
- Reaction KineticsDocument97 pagesReaction KineticsMuhammad Khuzaifah AzliNo ratings yet
- Changing The Rate of A Reaction (Part I)Document2 pagesChanging The Rate of A Reaction (Part I)Shahid Ur RehmanNo ratings yet
- Unit 4 Review Reaction Rates Answers To ReviewDocument8 pagesUnit 4 Review Reaction Rates Answers To ReviewANGELYN SANTOSNo ratings yet
- A00830666 - Lab Report - BatchDocument13 pagesA00830666 - Lab Report - BatchJocelyn garcia gonzalezNo ratings yet
- Determining The Order of A Chemical Reaction LabDocument8 pagesDetermining The Order of A Chemical Reaction LabJonathan_Khan7100% (1)
- Heat of NeutralisationDocument3 pagesHeat of NeutralisationCynthia RoneyNo ratings yet
- Chemical Exp 8Document10 pagesChemical Exp 8门门No ratings yet
- T2 - E5-Phase Transfer CatalystDocument14 pagesT2 - E5-Phase Transfer Catalystabhisheknemade9765No ratings yet
- CHE1700Document9 pagesCHE1700rachzammit2003No ratings yet
- Labsheet No.1: Kashif Husaain Sp-18-Bschem-004Document6 pagesLabsheet No.1: Kashif Husaain Sp-18-Bschem-004Muyammad Ahsan abbasNo ratings yet
- Year 10 Semester One Revision Sheet 6 ANSWERSDocument4 pagesYear 10 Semester One Revision Sheet 6 ANSWERSsports.kingdomNo ratings yet
- Purpose: Lab Work 1. Chemical Kinetics. Chemical EquilibriumDocument5 pagesPurpose: Lab Work 1. Chemical Kinetics. Chemical EquilibriumFritz ManyauNo ratings yet
- Experimental Report PDFDocument8 pagesExperimental Report PDFChrisNo ratings yet
- Ex.3-Heat of NeutralizationDocument10 pagesEx.3-Heat of Neutralizationalia2003skNo ratings yet
- 2012fall 113 FinalDocument15 pages2012fall 113 FinalSrynnENo ratings yet
- Data Sheet For Exp 1Document2 pagesData Sheet For Exp 1DanaNo ratings yet
- Benzoic - Acid ExperimentDocument7 pagesBenzoic - Acid ExperimentShivani SinghNo ratings yet
- Tutorial-Manual CH1002Document18 pagesTutorial-Manual CH1002Gift Chulu100% (2)
- Topic 6 16 MC PracticeDocument25 pagesTopic 6 16 MC PracticeDharmesh Ramnarayan YadavNo ratings yet
- 61-Rate of Reaction QP o Level - Cie - ChemistryDocument21 pages61-Rate of Reaction QP o Level - Cie - ChemistryZaid BabarNo ratings yet
- Appendix V L. Determination of PH Values - British PharmacopoeiaDocument3 pagesAppendix V L. Determination of PH Values - British PharmacopoeiaAbd El-Rahman SayedNo ratings yet
- 2020 - Equilibrium Practice ProblemsDocument5 pages2020 - Equilibrium Practice ProblemsAMOS SODJAHINNo ratings yet
- 2013 Usnco Local SolutionsDocument10 pages2013 Usnco Local SolutionsPelatihan KimiaNo ratings yet
- Practical LabDocument7 pagesPractical Labdaudiali2002No ratings yet
- Rate of ReactionDocument12 pagesRate of Reactionsurvival vlogsNo ratings yet
- Journal of Colligative PropertiesDocument9 pagesJournal of Colligative PropertiesMuhammad Baihaqi100% (1)
- Density of LiquidsDocument17 pagesDensity of LiquidsISAYA KICHELENo ratings yet
- Ap Unit7 WorksheetDocument4 pagesAp Unit7 Worksheetburcak gecNo ratings yet
- Ex 6Document2 pagesEx 63a2dNo ratings yet
- Ex 7Document3 pagesEx 73a2dNo ratings yet
- Ex 5Document2 pagesEx 53a2dNo ratings yet
- Ex 3Document2 pagesEx 33a2dNo ratings yet
- Ex 4Document2 pagesEx 43a2dNo ratings yet
- Experiment 2: "Determination of Boiling and Melting Points of Chemical Compounds" I. Observations and ResultsDocument2 pagesExperiment 2: "Determination of Boiling and Melting Points of Chemical Compounds" I. Observations and Results3a2dNo ratings yet
- Objectives:: AC Steady State PowerDocument5 pagesObjectives:: AC Steady State Power3a2dNo ratings yet
- Ex 1Document3 pagesEx 13a2d0% (1)
- Experiement 6Document3 pagesExperiement 63a2dNo ratings yet
- Experiement 5Document9 pagesExperiement 53a2dNo ratings yet
- Exp 9Document6 pagesExp 93a2dNo ratings yet
- Objective:: Electric LabDocument5 pagesObjective:: Electric Lab3a2dNo ratings yet
- Objective:: Electric LabDocument3 pagesObjective:: Electric Lab3a2dNo ratings yet
- مقدمةDocument1 pageمقدمة3a2dNo ratings yet
- Electric Lab .: The Basic Resistance MeasurementsDocument6 pagesElectric Lab .: The Basic Resistance Measurements3a2dNo ratings yet
- Objective:: Electric LabDocument8 pagesObjective:: Electric Lab3a2dNo ratings yet
- CyclamenDocument9 pagesCyclamenLAUM1No ratings yet
- Ideas and Methods of Turbomachinery Aerodynamics A Historical ViewDocument12 pagesIdeas and Methods of Turbomachinery Aerodynamics A Historical ViewLos MagandosNo ratings yet
- SOCIOLOGICAL FOUNDATION ReportDocument14 pagesSOCIOLOGICAL FOUNDATION Reportᜃᜒᜈ᜔ᜎᜒ ᜇᜒᜋᜈ᜔ᜇᜒᜋᜈ᜔No ratings yet
- Amity: UniversityDocument10 pagesAmity: UniversityVinayak MangotraNo ratings yet
- Unit-1 Introduction To Management and OrganizationsDocument73 pagesUnit-1 Introduction To Management and OrganizationsGnanesh GNo ratings yet
- Metriso 5000ADocument14 pagesMetriso 5000Ahellfire22000No ratings yet
- AIT Unit1 InternetDocument44 pagesAIT Unit1 InternetNaina_Dwivedi_6514No ratings yet
- Fundmentals Energy ProcessDocument11 pagesFundmentals Energy ProcessepjxNo ratings yet
- Postgraduate Prospectus For 2014 EntryDocument296 pagesPostgraduate Prospectus For 2014 EntryDaniel LieNo ratings yet
- 6chefs Plate - Fresh Ingredients & Delicious Recipes Delivered To Your DoorDocument6 pages6chefs Plate - Fresh Ingredients & Delicious Recipes Delivered To Your DoorEmmaNo ratings yet
- Why Electrical EngineeringDocument31 pagesWhy Electrical Engineeringsyed42210No ratings yet
- Eastern Europe ManufacturingDocument14 pagesEastern Europe ManufacturingNB Thushara HarithasNo ratings yet
- PDF Data Science and Machine Learning Mathematical and Statistical Methods Chapman Hall CRC Machine Learning Pattern Recognition 1St Edition Dirk P Kroese Ebook Full ChapterDocument54 pagesPDF Data Science and Machine Learning Mathematical and Statistical Methods Chapman Hall CRC Machine Learning Pattern Recognition 1St Edition Dirk P Kroese Ebook Full Chaptercatherine.cottingham887100% (5)
- SKYPAD Pocket Firmware Instructions PDFDocument3 pagesSKYPAD Pocket Firmware Instructions PDFAbelito FloresNo ratings yet
- Engels As Interpreter of Marx's EconomicsDocument37 pagesEngels As Interpreter of Marx's EconomicsJuan HermidasNo ratings yet
- Answer Keys For Problem Set 3: MIT 14.04 Intermediate Microeconomic Theory Fall 2003Document4 pagesAnswer Keys For Problem Set 3: MIT 14.04 Intermediate Microeconomic Theory Fall 2003Flavio Andrés Eichin CamposNo ratings yet
- ASUS RT-AC58U ManualDocument122 pagesASUS RT-AC58U ManualSeungpyo HongNo ratings yet
- Design Simulation and Realization of Solar Battery Charge Controller Using Arduino UnoDocument5 pagesDesign Simulation and Realization of Solar Battery Charge Controller Using Arduino UnoRushiraj DesaiNo ratings yet
- Department of Education: A. Background Information For Learners B. Most Essential Learning CompetencyDocument9 pagesDepartment of Education: A. Background Information For Learners B. Most Essential Learning CompetencyCamille CaigaNo ratings yet
- Infectious Diseases 2022 Virtual Abstract BookDocument53 pagesInfectious Diseases 2022 Virtual Abstract BookSriNo ratings yet
- V. Evaporator Design 1. Evaporator and Water Treatment EvaporatorDocument3 pagesV. Evaporator Design 1. Evaporator and Water Treatment EvaporatorEngelbert AntodNo ratings yet
- MCDonald's StrategyDocument3 pagesMCDonald's StrategyShaukat HasanNo ratings yet
- Sarah Ribeiro ResumeDocument1 pageSarah Ribeiro ResumeSarah RibeiroNo ratings yet
- MTTRAPD Huaweii2000 cl2Document3,295 pagesMTTRAPD Huaweii2000 cl2Muhammad AdnanNo ratings yet
- Assignment 2 Lesson Plan AnalysisDocument14 pagesAssignment 2 Lesson Plan Analysisapi-373008418No ratings yet
- His 418 SinenkosiDocument7 pagesHis 418 Sinenkosisiphiwo dlaminiNo ratings yet
- Fiqh of MarriageDocument30 pagesFiqh of MarriageNawaid ZamanNo ratings yet
- Guru 1 - 5 Whats NewDocument19 pagesGuru 1 - 5 Whats NewLuca RosacutaNo ratings yet