Professional Documents
Culture Documents
Emmision Cal STP Conditon
Emmision Cal STP Conditon
Uploaded by
mvbm31Copyright:
Available Formats
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5822)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Pump MaintenanceDocument9 pagesPump Maintenancemvbm3150% (2)
- Draft: The Calculation of Exhaust Gas DraftDocument2 pagesDraft: The Calculation of Exhaust Gas Draftmvbm31No ratings yet
- Chemical Cost at YR For Week 13Document4 pagesChemical Cost at YR For Week 13mvbm31No ratings yet
- Eye Safety Some Common Causes of Eye Injuries Different Kinds of Eye Protection Eye Safety Quiz ConclusionDocument6 pagesEye Safety Some Common Causes of Eye Injuries Different Kinds of Eye Protection Eye Safety Quiz Conclusionmvbm31No ratings yet
- 0.8 Density 48.6119 lb/ft3 780 kg/m3 Velocity 6.6 Ft/s 2 M/s SURGE PR 256.6708 PSIDocument1 page0.8 Density 48.6119 lb/ft3 780 kg/m3 Velocity 6.6 Ft/s 2 M/s SURGE PR 256.6708 PSImvbm31No ratings yet
- Rakesh Jhunjhunwala Portfolio, Holdings, Stock, SharesDocument4 pagesRakesh Jhunjhunwala Portfolio, Holdings, Stock, Sharesmvbm31No ratings yet
- Retail StocksDocument3 pagesRetail Stocksmvbm31No ratings yet
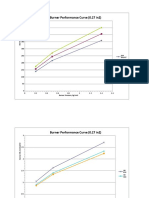
- Burner RatingDocument11 pagesBurner Ratingmvbm31No ratings yet
- One Set of FootprintsDocument1 pageOne Set of Footprintsmvbm31No ratings yet
Emmision Cal STP Conditon
Emmision Cal STP Conditon
Uploaded by
mvbm31Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Emmision Cal STP Conditon
Emmision Cal STP Conditon
Uploaded by
mvbm31Copyright:
Available Formats
STP conditions
[mg/Nm3] =
[ppm]
M mg
-------------22.4 ml
[ppm] =
[mg/Nm3] x
22.4 ml
-------------M mg
M[g] ---> 22.4l
1ml =
M
------mg
22.4
M[mg] ---> 22.4ml
1mg =
22.4
------ml
M
S.T.P conditons(Standard temperature and pressure conditions)
Ambient temp.
Barometric presssure
Volume
Standard O2 %
H2O
Converting O2 %
1 NOx
M= N(14g)+O2(32)=
M: Molecular weight for NO2
2 SOx
M= S(32g)+O2(32)=
M: Molecular weight as SO2
3 CO
mg/Nm3 at dry basis
107 [mg/Nm3] x 22.4 ml
46 mg
0
1
22.4
15
4.9
0
/M=
bar
ml
vol %
vol %
vol %
ppm
52 dry
50 wet
INPUT DATA
NO TOUCH
wet
based on
13.2 %O2
ppm
140 wet based on
148 dry
0.0 %O2
1114 [mg/Nm3] x
64 mg
22.4 ml
/M=
390 dry
371 wet
based on
13.2 %O2
1,050 wet based on
1,104 dry
0.0 %O2
119 [mg/Nm3] x
22.4 ml
/M=
95 dry
based on
13.2 %O2
256 wet based on
0.0 %O2
STP conditions
M= C(12g)+O2(16)=
M: Molecular weight as CO
4 HC
M= C(12g)+H4(4)=
M: Molecular weight as CH4
28 mg
91 wet
270 dry
182 [mg/Nm3] x
16 mg
22.4 ml
/M=
255 dry
242 wet
based on
13.2 %O2
686 wet based on
721 dry
0.0 %O2
5 PM(TSP)
185 [mg/Nm3] x
M= 0.21 x 32(O2) + 0.79 x 28(N2)=
29 mg
M: Molecular weight as Air(21 % O2+ 79% N)
22.4 ml
/M=
143 dry
136 wet
based on
13.2 %O2
385 wet based on
405 dry
0.0 %O2
182
22.4
16
254.8
20 [mg/Nm3]
22
64 mg
7
STP conditions
STP conditions
STP conditions
STP conditions
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5822)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Pump MaintenanceDocument9 pagesPump Maintenancemvbm3150% (2)
- Draft: The Calculation of Exhaust Gas DraftDocument2 pagesDraft: The Calculation of Exhaust Gas Draftmvbm31No ratings yet
- Chemical Cost at YR For Week 13Document4 pagesChemical Cost at YR For Week 13mvbm31No ratings yet
- Eye Safety Some Common Causes of Eye Injuries Different Kinds of Eye Protection Eye Safety Quiz ConclusionDocument6 pagesEye Safety Some Common Causes of Eye Injuries Different Kinds of Eye Protection Eye Safety Quiz Conclusionmvbm31No ratings yet
- 0.8 Density 48.6119 lb/ft3 780 kg/m3 Velocity 6.6 Ft/s 2 M/s SURGE PR 256.6708 PSIDocument1 page0.8 Density 48.6119 lb/ft3 780 kg/m3 Velocity 6.6 Ft/s 2 M/s SURGE PR 256.6708 PSImvbm31No ratings yet
- Rakesh Jhunjhunwala Portfolio, Holdings, Stock, SharesDocument4 pagesRakesh Jhunjhunwala Portfolio, Holdings, Stock, Sharesmvbm31No ratings yet
- Retail StocksDocument3 pagesRetail Stocksmvbm31No ratings yet
- Burner RatingDocument11 pagesBurner Ratingmvbm31No ratings yet
- One Set of FootprintsDocument1 pageOne Set of Footprintsmvbm31No ratings yet