Professional Documents
Culture Documents
Lecture 5 Problems
Lecture 5 Problems
Uploaded by
api-184918287Copyright:
Available Formats
You might also like
- Experiment 3 The Preparation of Potassium Tris (Oxalato) Ferrate (Iii) TrihydrateDocument9 pagesExperiment 3 The Preparation of Potassium Tris (Oxalato) Ferrate (Iii) Trihydrateainsovilinus0% (1)
- Either X-Ray Diffraction or (Infrared Spectroscopy)Document16 pagesEither X-Ray Diffraction or (Infrared Spectroscopy)Amna HaarisNo ratings yet
- Alcohols PDFDocument33 pagesAlcohols PDFDINESH DHANUSH KODINo ratings yet
- Chimie GB 2013 FinalDocument11 pagesChimie GB 2013 FinalChu Thi Hien ThuNo ratings yet
- Organic SimplificationDocument13 pagesOrganic SimplificationKaushal Silva RanpatabendigeNo ratings yet
- Chapter 11 12th Class 1Document22 pagesChapter 11 12th Class 1Nisha vankhadeNo ratings yet
- Chemistry Question With Solutions Imp For 12Document10 pagesChemistry Question With Solutions Imp For 12Himanshu GuptaNo ratings yet
- Important Questions of Grade 12 PDFDocument7 pagesImportant Questions of Grade 12 PDFBina NeupaneNo ratings yet
- Aldehydes Ketones and Carboxylic Acids - NCERT SolutionsDocument27 pagesAldehydes Ketones and Carboxylic Acids - NCERT SolutionsVyjayanthiNo ratings yet
- Grade 12 Karama Worksheet - AlcoholsDocument2 pagesGrade 12 Karama Worksheet - Alcoholsidk.raghavNo ratings yet
- Reactions of MonosaccharideDocument13 pagesReactions of MonosaccharideKate Lyle ParfanNo ratings yet
- Chemistry Ut2Document8 pagesChemistry Ut2vedantbaraskarNo ratings yet
- Chemistry Ut2Document8 pagesChemistry Ut2vedantbaraskarNo ratings yet
- 2005 RD 1 Questions tcm18-190744Document12 pages2005 RD 1 Questions tcm18-190744DeepMukherjeeNo ratings yet
- 2018 Hydroxy Cpds TutorialDocument4 pages2018 Hydroxy Cpds TutorialAmelia WongNo ratings yet
- Alcohols Phenols Ethers-1Document37 pagesAlcohols Phenols Ethers-1Subhransu Sekhar BarikNo ratings yet
- Organic Chemistry-1Document5 pagesOrganic Chemistry-1Milica RančićNo ratings yet
- Che QP 5Document20 pagesChe QP 5Shreeranga RbNo ratings yet
- Preparation of IodoformDocument18 pagesPreparation of IodoformHerminHardyantiUtami80% (5)
- Chemistry Test PaperDocument3 pagesChemistry Test PaperbrightcoachingacademyindiaNo ratings yet
- Alcohol, Phenol and EthersDocument7 pagesAlcohol, Phenol and EthersgreekyNo ratings yet
- AlcoholDocument2 pagesAlcoholVed patelNo ratings yet
- 2018 Hydroxy Cpds Tutorial SolutionDocument18 pages2018 Hydroxy Cpds Tutorial SolutionAmelia WongNo ratings yet
- Aliphatics Home PackageDocument6 pagesAliphatics Home PackageelishamahubiNo ratings yet
- Chapter 11 Alcohols Phenols and Ethers - Ncert Solutions: INTEXT QuestionsDocument39 pagesChapter 11 Alcohols Phenols and Ethers - Ncert Solutions: INTEXT QuestionsVyjayanthiNo ratings yet
- CBSE Sample Paper Class 12 Chemistry Set 6Document4 pagesCBSE Sample Paper Class 12 Chemistry Set 6Sidharth SabharwalNo ratings yet
- Alcohol, Phenol EtherDocument1 pageAlcohol, Phenol EtherSomu Yashawant ChaudhariNo ratings yet
- PhenolDocument32 pagesPhenolchithiraikumar83No ratings yet
- Chemistry Chapter 11 Alcohol, Phenol and EtherDocument32 pagesChemistry Chapter 11 Alcohol, Phenol and EtherVidyakulNo ratings yet
- ALCOHOLS, PHENOLS & ETHERS QuesDocument12 pagesALCOHOLS, PHENOLS & ETHERS Quesaryaveer376No ratings yet
- F324 June 2013 Unofficial Mark SchemeDocument7 pagesF324 June 2013 Unofficial Mark SchemearyoaudittNo ratings yet
- Monthly Test Xii Chemistry October 2023-24Document4 pagesMonthly Test Xii Chemistry October 2023-24soumityachaudharyNo ratings yet
- Alcohol, Phenol and EthersDocument28 pagesAlcohol, Phenol and EthersMohd ShareefNo ratings yet
- Hydroarbons: 1. Give The Different Confirmations of Ethane With Their Newman Projection FormulaDocument2 pagesHydroarbons: 1. Give The Different Confirmations of Ethane With Their Newman Projection FormulaAmogh SinghNo ratings yet
- Revision 8-Prelims mock-Chemistry-QDocument7 pagesRevision 8-Prelims mock-Chemistry-QARYA LIMAYENo ratings yet
- Alcohols Phenols EthersDocument34 pagesAlcohols Phenols Ethersmonithadhanabalan476No ratings yet
- CH 11 ExerciseDocument33 pagesCH 11 ExerciseTr Mazhar PunjabiNo ratings yet
- Science 2nd Term Test 2018 Provincial Past PapersDocument6 pagesScience 2nd Term Test 2018 Provincial Past PapersGunaa KajananNo ratings yet
- Model Paper With SolutionsDocument16 pagesModel Paper With SolutionsHoly GhostNo ratings yet
- Class XII Alcohols Phenols EthersDocument7 pagesClass XII Alcohols Phenols EthersvartikasinghNo ratings yet
- To Download Other Subject Question Bank Visit WWW - Eshaale.inDocument18 pagesTo Download Other Subject Question Bank Visit WWW - Eshaale.inSwetha RNNo ratings yet
- Model Question PapersDocument68 pagesModel Question PaperssanchitaNo ratings yet
- Edexcel - IAS - Organic Chemistry - 1Document27 pagesEdexcel - IAS - Organic Chemistry - 1mostafa barakatNo ratings yet
- SUMMER VACATION ASSIGNMENT OF CLASS XII (2023-2024)Document3 pagesSUMMER VACATION ASSIGNMENT OF CLASS XII (2023-2024)Mohammed RizwanNo ratings yet
- Grade Xii (Chemistry) : Aldehydes, Ketones and Carboxylic Acids (Term - 2) : Most Expecting QuestionsDocument5 pagesGrade Xii (Chemistry) : Aldehydes, Ketones and Carboxylic Acids (Term - 2) : Most Expecting QuestionsSupreeta KhatiwadaNo ratings yet
- Tamil Nadu State Board - Class XII Chemistry (Model Paper) : General Instructions: General InstructionsDocument9 pagesTamil Nadu State Board - Class XII Chemistry (Model Paper) : General Instructions: General InstructionsVishwath RamNo ratings yet
- Chemistry SyllabusDocument42 pagesChemistry SyllabusKartikey JainNo ratings yet
- 12th Ch.7 Alcohols Phenols and EthersDocument4 pages12th Ch.7 Alcohols Phenols and EthersjusttryingtoghostNo ratings yet
- Revision Test-1, 12th ChemistryDocument4 pagesRevision Test-1, 12th ChemistryVasanthakumar shanmugamNo ratings yet
- Alcohol, Phenols and EtherDocument4 pagesAlcohol, Phenols and EtherShayaan & friend's vlogNo ratings yet
- Alcohols, Phenols, Ethers - Board QuestionsDocument7 pagesAlcohols, Phenols, Ethers - Board QuestionsIron ManNo ratings yet
- Class: Xii Max. Marks: 50 Subject: Chemistry. TIME: 2 HoursDocument2 pagesClass: Xii Max. Marks: 50 Subject: Chemistry. TIME: 2 HoursPrerak Kumar SharmaNo ratings yet
- Lecture Questions CZB190Document18 pagesLecture Questions CZB190micro0908No ratings yet
- Answer Key Assignment No. 3 Chapters 11 12 (AY2021-2022 (2nd Sem)Document8 pagesAnswer Key Assignment No. 3 Chapters 11 12 (AY2021-2022 (2nd Sem)REGINE CUEVASNo ratings yet
- Por Jorge L: Uis Breña OréDocument32 pagesPor Jorge L: Uis Breña OréAlexa TorresNo ratings yet
- Test - Alcohols, Phenols and Ethers - 28.8.2023Document3 pagesTest - Alcohols, Phenols and Ethers - 28.8.2023jayaprasanthsinghNo ratings yet
- CoversionDocument12 pagesCoversionSunil KumarNo ratings yet
- Class 12 - Chemistry - Alcohols, Phenols and EthersDocument75 pagesClass 12 - Chemistry - Alcohols, Phenols and EthersFlash in AshishNo ratings yet
- ثانوية الصباح الرسمية - علوم حياة انكليزي - نهائي 2023-2024Document8 pagesثانوية الصباح الرسمية - علوم حياة انكليزي - نهائي 2023-2024abirtoukan0No ratings yet
- Practice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersFrom EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersRating: 3 out of 5 stars3/5 (2)
- Chapter - 1 Layout - DraftDocument8 pagesChapter - 1 Layout - Draftapi-184918287No ratings yet
- Lecture 6 ProblemsDocument2 pagesLecture 6 Problemsapi-184918287No ratings yet
- Lecture 7 ProblemsDocument1 pageLecture 7 Problemsapi-184918287No ratings yet
- Lecture 4 ProblemsDocument2 pagesLecture 4 Problemsapi-184918287No ratings yet
- DaviscvDocument4 pagesDaviscvapi-184918287No ratings yet
Lecture 5 Problems
Lecture 5 Problems
Uploaded by
api-184918287Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lecture 5 Problems
Lecture 5 Problems
Uploaded by
api-184918287Copyright:
Available Formats
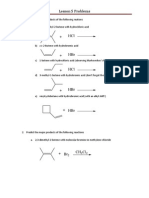
Lesson 5 Problems
1. Arrange the following nucleophiles in order of increasing nucleophilicity Ethoxide, hydroxide, water and amide ion 2. Arrange the following leaving groups in order of increasing stability Chloride, fluoride, bromide, hydroxide, water 3. Predict whether each of the reactions below will run by an SN1 or SN2 mechanism a) b) c) d) Potassium hydroxide reacting with methyl chloride Sodium amide reacting with ethyl bromide Ammonia reacting with t-butyl iodide Ethanol and isopropyl iodide
4. Phosphoric acid can be used to protonate alcohols, creating a situation with a good leaving group on a secondary carbon, but no nucleophile. Then 3-pentanol is mixed with phorphoric acid, what product is expected to form?
5. When cyclohexyl chloride is mixed with sodium methoxide, cyclohexene is produced. Draw a mechanism for this reaction.
6. When (2S, 2R)-2-bromo-34-methylpentane reacts with methoxide ion, only one of the two possible isomers forms. Circle the isomer which forms and explain this observation.
You might also like
- Experiment 3 The Preparation of Potassium Tris (Oxalato) Ferrate (Iii) TrihydrateDocument9 pagesExperiment 3 The Preparation of Potassium Tris (Oxalato) Ferrate (Iii) Trihydrateainsovilinus0% (1)
- Either X-Ray Diffraction or (Infrared Spectroscopy)Document16 pagesEither X-Ray Diffraction or (Infrared Spectroscopy)Amna HaarisNo ratings yet
- Alcohols PDFDocument33 pagesAlcohols PDFDINESH DHANUSH KODINo ratings yet
- Chimie GB 2013 FinalDocument11 pagesChimie GB 2013 FinalChu Thi Hien ThuNo ratings yet
- Organic SimplificationDocument13 pagesOrganic SimplificationKaushal Silva RanpatabendigeNo ratings yet
- Chapter 11 12th Class 1Document22 pagesChapter 11 12th Class 1Nisha vankhadeNo ratings yet
- Chemistry Question With Solutions Imp For 12Document10 pagesChemistry Question With Solutions Imp For 12Himanshu GuptaNo ratings yet
- Important Questions of Grade 12 PDFDocument7 pagesImportant Questions of Grade 12 PDFBina NeupaneNo ratings yet
- Aldehydes Ketones and Carboxylic Acids - NCERT SolutionsDocument27 pagesAldehydes Ketones and Carboxylic Acids - NCERT SolutionsVyjayanthiNo ratings yet
- Grade 12 Karama Worksheet - AlcoholsDocument2 pagesGrade 12 Karama Worksheet - Alcoholsidk.raghavNo ratings yet
- Reactions of MonosaccharideDocument13 pagesReactions of MonosaccharideKate Lyle ParfanNo ratings yet
- Chemistry Ut2Document8 pagesChemistry Ut2vedantbaraskarNo ratings yet
- Chemistry Ut2Document8 pagesChemistry Ut2vedantbaraskarNo ratings yet
- 2005 RD 1 Questions tcm18-190744Document12 pages2005 RD 1 Questions tcm18-190744DeepMukherjeeNo ratings yet
- 2018 Hydroxy Cpds TutorialDocument4 pages2018 Hydroxy Cpds TutorialAmelia WongNo ratings yet
- Alcohols Phenols Ethers-1Document37 pagesAlcohols Phenols Ethers-1Subhransu Sekhar BarikNo ratings yet
- Organic Chemistry-1Document5 pagesOrganic Chemistry-1Milica RančićNo ratings yet
- Che QP 5Document20 pagesChe QP 5Shreeranga RbNo ratings yet
- Preparation of IodoformDocument18 pagesPreparation of IodoformHerminHardyantiUtami80% (5)
- Chemistry Test PaperDocument3 pagesChemistry Test PaperbrightcoachingacademyindiaNo ratings yet
- Alcohol, Phenol and EthersDocument7 pagesAlcohol, Phenol and EthersgreekyNo ratings yet
- AlcoholDocument2 pagesAlcoholVed patelNo ratings yet
- 2018 Hydroxy Cpds Tutorial SolutionDocument18 pages2018 Hydroxy Cpds Tutorial SolutionAmelia WongNo ratings yet
- Aliphatics Home PackageDocument6 pagesAliphatics Home PackageelishamahubiNo ratings yet
- Chapter 11 Alcohols Phenols and Ethers - Ncert Solutions: INTEXT QuestionsDocument39 pagesChapter 11 Alcohols Phenols and Ethers - Ncert Solutions: INTEXT QuestionsVyjayanthiNo ratings yet
- CBSE Sample Paper Class 12 Chemistry Set 6Document4 pagesCBSE Sample Paper Class 12 Chemistry Set 6Sidharth SabharwalNo ratings yet
- Alcohol, Phenol EtherDocument1 pageAlcohol, Phenol EtherSomu Yashawant ChaudhariNo ratings yet
- PhenolDocument32 pagesPhenolchithiraikumar83No ratings yet
- Chemistry Chapter 11 Alcohol, Phenol and EtherDocument32 pagesChemistry Chapter 11 Alcohol, Phenol and EtherVidyakulNo ratings yet
- ALCOHOLS, PHENOLS & ETHERS QuesDocument12 pagesALCOHOLS, PHENOLS & ETHERS Quesaryaveer376No ratings yet
- F324 June 2013 Unofficial Mark SchemeDocument7 pagesF324 June 2013 Unofficial Mark SchemearyoaudittNo ratings yet
- Monthly Test Xii Chemistry October 2023-24Document4 pagesMonthly Test Xii Chemistry October 2023-24soumityachaudharyNo ratings yet
- Alcohol, Phenol and EthersDocument28 pagesAlcohol, Phenol and EthersMohd ShareefNo ratings yet
- Hydroarbons: 1. Give The Different Confirmations of Ethane With Their Newman Projection FormulaDocument2 pagesHydroarbons: 1. Give The Different Confirmations of Ethane With Their Newman Projection FormulaAmogh SinghNo ratings yet
- Revision 8-Prelims mock-Chemistry-QDocument7 pagesRevision 8-Prelims mock-Chemistry-QARYA LIMAYENo ratings yet
- Alcohols Phenols EthersDocument34 pagesAlcohols Phenols Ethersmonithadhanabalan476No ratings yet
- CH 11 ExerciseDocument33 pagesCH 11 ExerciseTr Mazhar PunjabiNo ratings yet
- Science 2nd Term Test 2018 Provincial Past PapersDocument6 pagesScience 2nd Term Test 2018 Provincial Past PapersGunaa KajananNo ratings yet
- Model Paper With SolutionsDocument16 pagesModel Paper With SolutionsHoly GhostNo ratings yet
- Class XII Alcohols Phenols EthersDocument7 pagesClass XII Alcohols Phenols EthersvartikasinghNo ratings yet
- To Download Other Subject Question Bank Visit WWW - Eshaale.inDocument18 pagesTo Download Other Subject Question Bank Visit WWW - Eshaale.inSwetha RNNo ratings yet
- Model Question PapersDocument68 pagesModel Question PaperssanchitaNo ratings yet
- Edexcel - IAS - Organic Chemistry - 1Document27 pagesEdexcel - IAS - Organic Chemistry - 1mostafa barakatNo ratings yet
- SUMMER VACATION ASSIGNMENT OF CLASS XII (2023-2024)Document3 pagesSUMMER VACATION ASSIGNMENT OF CLASS XII (2023-2024)Mohammed RizwanNo ratings yet
- Grade Xii (Chemistry) : Aldehydes, Ketones and Carboxylic Acids (Term - 2) : Most Expecting QuestionsDocument5 pagesGrade Xii (Chemistry) : Aldehydes, Ketones and Carboxylic Acids (Term - 2) : Most Expecting QuestionsSupreeta KhatiwadaNo ratings yet
- Tamil Nadu State Board - Class XII Chemistry (Model Paper) : General Instructions: General InstructionsDocument9 pagesTamil Nadu State Board - Class XII Chemistry (Model Paper) : General Instructions: General InstructionsVishwath RamNo ratings yet
- Chemistry SyllabusDocument42 pagesChemistry SyllabusKartikey JainNo ratings yet
- 12th Ch.7 Alcohols Phenols and EthersDocument4 pages12th Ch.7 Alcohols Phenols and EthersjusttryingtoghostNo ratings yet
- Revision Test-1, 12th ChemistryDocument4 pagesRevision Test-1, 12th ChemistryVasanthakumar shanmugamNo ratings yet
- Alcohol, Phenols and EtherDocument4 pagesAlcohol, Phenols and EtherShayaan & friend's vlogNo ratings yet
- Alcohols, Phenols, Ethers - Board QuestionsDocument7 pagesAlcohols, Phenols, Ethers - Board QuestionsIron ManNo ratings yet
- Class: Xii Max. Marks: 50 Subject: Chemistry. TIME: 2 HoursDocument2 pagesClass: Xii Max. Marks: 50 Subject: Chemistry. TIME: 2 HoursPrerak Kumar SharmaNo ratings yet
- Lecture Questions CZB190Document18 pagesLecture Questions CZB190micro0908No ratings yet
- Answer Key Assignment No. 3 Chapters 11 12 (AY2021-2022 (2nd Sem)Document8 pagesAnswer Key Assignment No. 3 Chapters 11 12 (AY2021-2022 (2nd Sem)REGINE CUEVASNo ratings yet
- Por Jorge L: Uis Breña OréDocument32 pagesPor Jorge L: Uis Breña OréAlexa TorresNo ratings yet
- Test - Alcohols, Phenols and Ethers - 28.8.2023Document3 pagesTest - Alcohols, Phenols and Ethers - 28.8.2023jayaprasanthsinghNo ratings yet
- CoversionDocument12 pagesCoversionSunil KumarNo ratings yet
- Class 12 - Chemistry - Alcohols, Phenols and EthersDocument75 pagesClass 12 - Chemistry - Alcohols, Phenols and EthersFlash in AshishNo ratings yet
- ثانوية الصباح الرسمية - علوم حياة انكليزي - نهائي 2023-2024Document8 pagesثانوية الصباح الرسمية - علوم حياة انكليزي - نهائي 2023-2024abirtoukan0No ratings yet
- Practice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersFrom EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersRating: 3 out of 5 stars3/5 (2)
- Chapter - 1 Layout - DraftDocument8 pagesChapter - 1 Layout - Draftapi-184918287No ratings yet
- Lecture 6 ProblemsDocument2 pagesLecture 6 Problemsapi-184918287No ratings yet
- Lecture 7 ProblemsDocument1 pageLecture 7 Problemsapi-184918287No ratings yet
- Lecture 4 ProblemsDocument2 pagesLecture 4 Problemsapi-184918287No ratings yet
- DaviscvDocument4 pagesDaviscvapi-184918287No ratings yet