Professional Documents
Culture Documents
Session #34: Homework Problems: Problem #1
Session #34: Homework Problems: Problem #1
Uploaded by
Mohamed Azlan SuhotCopyright:
Available Formats
You might also like
- Chapt 08Document21 pagesChapt 08Jesse McClure100% (5)
- Assignments On Phase Equilibrium in CeramicsDocument5 pagesAssignments On Phase Equilibrium in CeramicsDESALEGN SHIBESHNo ratings yet
- TYPD ExercisesDocument10 pagesTYPD ExercisesConstance Lynn'da GNo ratings yet
- Mse SW2CDocument11 pagesMse SW2CmarkkkkkNo ratings yet
- Question & Answer Set-7Document12 pagesQuestion & Answer Set-7eeng.ali651550% (2)
- MIT3 091SCF09 hw34 SolDocument7 pagesMIT3 091SCF09 hw34 SolNgoc NguyenNo ratings yet
- Tutorial 7 - Phase DiagramsDocument4 pagesTutorial 7 - Phase DiagramsSYAFIQAH ISMAILNo ratings yet
- EG 244 Assignment 3 Phase DiagramDocument3 pagesEG 244 Assignment 3 Phase DiagramGoodson KolalaNo ratings yet
- Chem PPRDocument4 pagesChem PPRJitendra KaushikNo ratings yet
- Animated Figure 9.20: SketchDocument11 pagesAnimated Figure 9.20: SketchmarkkkkkNo ratings yet
- Assignment PhaseDiaDocument5 pagesAssignment PhaseDiaAnshu Kumar GuptaNo ratings yet
- Tutorial 3 SolutionDocument5 pagesTutorial 3 SolutionUmmi Kalthum MohamadNo ratings yet
- 240 E19Document17 pages240 E19kimiaNo ratings yet
- Sheet 2 (Material)Document6 pagesSheet 2 (Material)Omar AssalNo ratings yet
- Chapter8 PhaseDiagram HandoutsDocument27 pagesChapter8 PhaseDiagram Handoutswagdy87No ratings yet
- A Level Chemistry Paper 2 Exam 6Document4 pagesA Level Chemistry Paper 2 Exam 6majanga johnNo ratings yet
- 104 PhaseDiags QS2AnsDocument6 pages104 PhaseDiags QS2Ansnilanga123No ratings yet
- 2021 August CH204-HDocument3 pages2021 August CH204-HMidhunNo ratings yet
- Closed-Book Practice-Ch 09 (2017!08!07)Document9 pagesClosed-Book Practice-Ch 09 (2017!08!07)Juan100% (1)
- Practice Questions On CHM 212Document4 pagesPractice Questions On CHM 212Help HandNo ratings yet
- Cre HandoutsDocument21 pagesCre HandoutsPinak DattarayNo ratings yet
- CBE 141-Chemical Engineering Thermodynamics Spring 2015Document4 pagesCBE 141-Chemical Engineering Thermodynamics Spring 2015GuntherNo ratings yet
- Ch-27.3 Iron Carbon Equilibrium DiagramDocument20 pagesCh-27.3 Iron Carbon Equilibrium DiagramasjfgauojfgfNo ratings yet
- Samp 103151 Exam V2 S210Document15 pagesSamp 103151 Exam V2 S210gpeck92No ratings yet
- Eutectic Mixtures: Similarities Between Eutectic Mixture and Pure CompoundDocument8 pagesEutectic Mixtures: Similarities Between Eutectic Mixture and Pure CompoundNaduku EridadNo ratings yet
- Gujarat Technological UniversityDocument2 pagesGujarat Technological Universityfeyayel988No ratings yet
- A Level Chemistry Paper 2 Exam 20Document6 pagesA Level Chemistry Paper 2 Exam 20Anthony AndyNo ratings yet
- ALEVELREVISIONQUESTIONSDocument7 pagesALEVELREVISIONQUESTIONSAnthony AndyNo ratings yet
- JUNE 2013 Section A: Physical and General Chemistry (Answer Only TWO Questions in This Section)Document10 pagesJUNE 2013 Section A: Physical and General Chemistry (Answer Only TWO Questions in This Section)Zozo FozaoNo ratings yet
- Chem Cgce 2011 A/lDocument9 pagesChem Cgce 2011 A/lmengotNo ratings yet
- CH 10Document38 pagesCH 10Vamsi KrishnaNo ratings yet
- Phase DiagramDocument47 pagesPhase DiagramMuhammed MansNo ratings yet
- Chemistry 2Document3 pagesChemistry 2daudimgetamafweleNo ratings yet
- 2022 Uace Chemistry SeminarDocument11 pages2022 Uace Chemistry Seminarmakueimadol17No ratings yet
- KAMAL Assignment July Phy&ChemDocument3 pagesKAMAL Assignment July Phy&ChemSHERIFF LAWALNo ratings yet
- Chapter 8 - Phase Diagram PART2Document26 pagesChapter 8 - Phase Diagram PART2Mohd IqbalNo ratings yet
- MLE1101 - Tutorial 4 - Suggested SolutionsDocument7 pagesMLE1101 - Tutorial 4 - Suggested SolutionsYin HauNo ratings yet
- Chapter 06 Phase EquilibriaDocument77 pagesChapter 06 Phase Equilibriaakimarf60% (5)
- Sheet 5 - Phase Diagrams IIDocument2 pagesSheet 5 - Phase Diagrams IIroro98syamNo ratings yet
- 2020 Che 450 Assignment 2Document2 pages2020 Che 450 Assignment 2Lad SlasNo ratings yet
- Materials Science - Alloys System - 2Document16 pagesMaterials Science - Alloys System - 2Sumit JoshiNo ratings yet
- JJKDocument11 pagesJJKAnonymous pa8pSCC15No ratings yet
- Che 3330 - Spring 2012 HW 5Document5 pagesChe 3330 - Spring 2012 HW 5Brett CasserlyNo ratings yet
- CH3 Questions: Fig. 10-20 Unary Phase Diagram For Carbon. (Region For Diamond Formation Is Shown With A Dotted Line)Document4 pagesCH3 Questions: Fig. 10-20 Unary Phase Diagram For Carbon. (Region For Diamond Formation Is Shown With A Dotted Line)Mohammed IbrahimNo ratings yet
- Chemistry 1Document5 pagesChemistry 1youngtillionez99No ratings yet
- Syllabus Tuto DK014Document5 pagesSyllabus Tuto DK014Chem MistryNo ratings yet
- B49CE Exam Answers v1Document40 pagesB49CE Exam Answers v1Konul AlizadehNo ratings yet
- CFD (AE2402) Question BankDocument6 pagesCFD (AE2402) Question Bankaeroacademic50% (2)
- PIKEMDocument2 pagesPIKEMDream CakeNo ratings yet
- Chapter 9: Phase Diagrams: Issues To Address..Document38 pagesChapter 9: Phase Diagrams: Issues To Address..yunlu0705No ratings yet
- 練習單3 2Document8 pages練習單3 2Marco RezendeNo ratings yet
- MCQ Phase DiagramsDocument3 pagesMCQ Phase DiagramsPrince AbhishekNo ratings yet
- CHM 471 Tutorial 3 Phase DiagramDocument4 pagesCHM 471 Tutorial 3 Phase DiagramCharlesRolendNo ratings yet
- Chemistry F4 Set 13 14Document7 pagesChemistry F4 Set 13 14samwelaldoboyNo ratings yet
- Attainable Region Theory: An Introduction to Choosing an Optimal ReactorFrom EverandAttainable Region Theory: An Introduction to Choosing an Optimal ReactorNo ratings yet
- Reviews in Computational ChemistryFrom EverandReviews in Computational ChemistryAbby L. ParrillNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Advanced Battery MaterialsFrom EverandAdvanced Battery MaterialsChunwen SunNo ratings yet
Session #34: Homework Problems: Problem #1
Session #34: Homework Problems: Problem #1
Uploaded by
Mohamed Azlan SuhotOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Session #34: Homework Problems: Problem #1
Session #34: Homework Problems: Problem #1
Uploaded by
Mohamed Azlan SuhotCopyright:
Available Formats
Session #34: Homework Problems
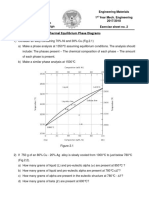
Problem #1 For the binary system Cu-Ni the following data are available from cooling experiments:
composition of solid first formed on cooling (atomic % Ni)
T(C)
melt composition (atomic % Ni)
1100 1180 1260 1340 1410
3 20 40 60 80
10 37 57 73 87
(a) From these data and information provided in the Periodic Table, construct the phase diagram (T vs c). (b) At each of the following (T,c) coordinates, i.e., combinations of temperature and composition, what are the phases present and what are their respective compositions?
T( C)
composition (atomic % Ni)
1120 1200 1300
15 55 60
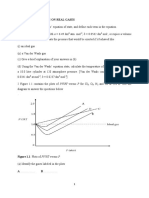
Problem #2 Draw schematic phase diagrams for binary systems with (a) complete liquid and solid solubility, (b) complete liquid but zero solid solubility, and (c) complete liquid and limited solid solubility. (In your sketches label phase fields and give characteristic temperatures.) Problem #3 The Cs-K phase diagram is given on the next page. Refer to it in answering the following questions.
(a) For a sample of composition 20 at. % Cs and 80 wt. % K held at 10C, determine (i) the composition of the solid phase present at equilibrium (ii) the composition of the liquid phase present at equilibrium (iii) the relative amounts of solid and liquid phases present at equilibrium (b) For a sample of composition 50 at % Cs and 50 at % K held at -37.5C, (i) what phases are present at equilibrium? (ii) what is the composition of each phase?
source unknown. All rights reserved. This content is excluded from our Creative Commons license. For more information, see http://ocw.mit.edu/fairuse.
Problem #4 (a) At each of the following coordinates on the binary phase diagram of NaCl H2O (see next page) at 1 atmosphere pressure, (i) identify the stable phases, (ii) give their compositions, and (iii) calculate their relative proportions: (1) 10% NaCl, 0oC (2) 10% NaCl, -10oC (3) 10% NaCl, -25oC
(b) For a solution of 23.3% NaCl in H2O at 21C, (i) identify the stable phases present, and (ii) give the composition of each phase
MIT OpenCourseWare http://ocw.mit.edu
3.091SC Introduction to Solid State Chemistry
Fall 2009
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
You might also like
- Chapt 08Document21 pagesChapt 08Jesse McClure100% (5)
- Assignments On Phase Equilibrium in CeramicsDocument5 pagesAssignments On Phase Equilibrium in CeramicsDESALEGN SHIBESHNo ratings yet
- TYPD ExercisesDocument10 pagesTYPD ExercisesConstance Lynn'da GNo ratings yet
- Mse SW2CDocument11 pagesMse SW2CmarkkkkkNo ratings yet
- Question & Answer Set-7Document12 pagesQuestion & Answer Set-7eeng.ali651550% (2)
- MIT3 091SCF09 hw34 SolDocument7 pagesMIT3 091SCF09 hw34 SolNgoc NguyenNo ratings yet
- Tutorial 7 - Phase DiagramsDocument4 pagesTutorial 7 - Phase DiagramsSYAFIQAH ISMAILNo ratings yet
- EG 244 Assignment 3 Phase DiagramDocument3 pagesEG 244 Assignment 3 Phase DiagramGoodson KolalaNo ratings yet
- Chem PPRDocument4 pagesChem PPRJitendra KaushikNo ratings yet
- Animated Figure 9.20: SketchDocument11 pagesAnimated Figure 9.20: SketchmarkkkkkNo ratings yet
- Assignment PhaseDiaDocument5 pagesAssignment PhaseDiaAnshu Kumar GuptaNo ratings yet
- Tutorial 3 SolutionDocument5 pagesTutorial 3 SolutionUmmi Kalthum MohamadNo ratings yet
- 240 E19Document17 pages240 E19kimiaNo ratings yet
- Sheet 2 (Material)Document6 pagesSheet 2 (Material)Omar AssalNo ratings yet
- Chapter8 PhaseDiagram HandoutsDocument27 pagesChapter8 PhaseDiagram Handoutswagdy87No ratings yet
- A Level Chemistry Paper 2 Exam 6Document4 pagesA Level Chemistry Paper 2 Exam 6majanga johnNo ratings yet
- 104 PhaseDiags QS2AnsDocument6 pages104 PhaseDiags QS2Ansnilanga123No ratings yet
- 2021 August CH204-HDocument3 pages2021 August CH204-HMidhunNo ratings yet
- Closed-Book Practice-Ch 09 (2017!08!07)Document9 pagesClosed-Book Practice-Ch 09 (2017!08!07)Juan100% (1)
- Practice Questions On CHM 212Document4 pagesPractice Questions On CHM 212Help HandNo ratings yet
- Cre HandoutsDocument21 pagesCre HandoutsPinak DattarayNo ratings yet
- CBE 141-Chemical Engineering Thermodynamics Spring 2015Document4 pagesCBE 141-Chemical Engineering Thermodynamics Spring 2015GuntherNo ratings yet
- Ch-27.3 Iron Carbon Equilibrium DiagramDocument20 pagesCh-27.3 Iron Carbon Equilibrium DiagramasjfgauojfgfNo ratings yet
- Samp 103151 Exam V2 S210Document15 pagesSamp 103151 Exam V2 S210gpeck92No ratings yet
- Eutectic Mixtures: Similarities Between Eutectic Mixture and Pure CompoundDocument8 pagesEutectic Mixtures: Similarities Between Eutectic Mixture and Pure CompoundNaduku EridadNo ratings yet
- Gujarat Technological UniversityDocument2 pagesGujarat Technological Universityfeyayel988No ratings yet
- A Level Chemistry Paper 2 Exam 20Document6 pagesA Level Chemistry Paper 2 Exam 20Anthony AndyNo ratings yet
- ALEVELREVISIONQUESTIONSDocument7 pagesALEVELREVISIONQUESTIONSAnthony AndyNo ratings yet
- JUNE 2013 Section A: Physical and General Chemistry (Answer Only TWO Questions in This Section)Document10 pagesJUNE 2013 Section A: Physical and General Chemistry (Answer Only TWO Questions in This Section)Zozo FozaoNo ratings yet
- Chem Cgce 2011 A/lDocument9 pagesChem Cgce 2011 A/lmengotNo ratings yet
- CH 10Document38 pagesCH 10Vamsi KrishnaNo ratings yet
- Phase DiagramDocument47 pagesPhase DiagramMuhammed MansNo ratings yet
- Chemistry 2Document3 pagesChemistry 2daudimgetamafweleNo ratings yet
- 2022 Uace Chemistry SeminarDocument11 pages2022 Uace Chemistry Seminarmakueimadol17No ratings yet
- KAMAL Assignment July Phy&ChemDocument3 pagesKAMAL Assignment July Phy&ChemSHERIFF LAWALNo ratings yet
- Chapter 8 - Phase Diagram PART2Document26 pagesChapter 8 - Phase Diagram PART2Mohd IqbalNo ratings yet
- MLE1101 - Tutorial 4 - Suggested SolutionsDocument7 pagesMLE1101 - Tutorial 4 - Suggested SolutionsYin HauNo ratings yet
- Chapter 06 Phase EquilibriaDocument77 pagesChapter 06 Phase Equilibriaakimarf60% (5)
- Sheet 5 - Phase Diagrams IIDocument2 pagesSheet 5 - Phase Diagrams IIroro98syamNo ratings yet
- 2020 Che 450 Assignment 2Document2 pages2020 Che 450 Assignment 2Lad SlasNo ratings yet
- Materials Science - Alloys System - 2Document16 pagesMaterials Science - Alloys System - 2Sumit JoshiNo ratings yet
- JJKDocument11 pagesJJKAnonymous pa8pSCC15No ratings yet
- Che 3330 - Spring 2012 HW 5Document5 pagesChe 3330 - Spring 2012 HW 5Brett CasserlyNo ratings yet
- CH3 Questions: Fig. 10-20 Unary Phase Diagram For Carbon. (Region For Diamond Formation Is Shown With A Dotted Line)Document4 pagesCH3 Questions: Fig. 10-20 Unary Phase Diagram For Carbon. (Region For Diamond Formation Is Shown With A Dotted Line)Mohammed IbrahimNo ratings yet
- Chemistry 1Document5 pagesChemistry 1youngtillionez99No ratings yet
- Syllabus Tuto DK014Document5 pagesSyllabus Tuto DK014Chem MistryNo ratings yet
- B49CE Exam Answers v1Document40 pagesB49CE Exam Answers v1Konul AlizadehNo ratings yet
- CFD (AE2402) Question BankDocument6 pagesCFD (AE2402) Question Bankaeroacademic50% (2)
- PIKEMDocument2 pagesPIKEMDream CakeNo ratings yet
- Chapter 9: Phase Diagrams: Issues To Address..Document38 pagesChapter 9: Phase Diagrams: Issues To Address..yunlu0705No ratings yet
- 練習單3 2Document8 pages練習單3 2Marco RezendeNo ratings yet
- MCQ Phase DiagramsDocument3 pagesMCQ Phase DiagramsPrince AbhishekNo ratings yet
- CHM 471 Tutorial 3 Phase DiagramDocument4 pagesCHM 471 Tutorial 3 Phase DiagramCharlesRolendNo ratings yet
- Chemistry F4 Set 13 14Document7 pagesChemistry F4 Set 13 14samwelaldoboyNo ratings yet
- Attainable Region Theory: An Introduction to Choosing an Optimal ReactorFrom EverandAttainable Region Theory: An Introduction to Choosing an Optimal ReactorNo ratings yet
- Reviews in Computational ChemistryFrom EverandReviews in Computational ChemistryAbby L. ParrillNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Advanced Battery MaterialsFrom EverandAdvanced Battery MaterialsChunwen SunNo ratings yet