Professional Documents
Culture Documents
Distillation: 8: Distillate/receiving Flask 9: Vacuum/gas Inlet 10: Still Receiver 11: Heat Control 12: Stirrer
Distillation: 8: Distillate/receiving Flask 9: Vacuum/gas Inlet 10: Still Receiver 11: Heat Control 12: Stirrer
Uploaded by
Subhash DhungelOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Distillation: 8: Distillate/receiving Flask 9: Vacuum/gas Inlet 10: Still Receiver 11: Heat Control 12: Stirrer
Distillation: 8: Distillate/receiving Flask 9: Vacuum/gas Inlet 10: Still Receiver 11: Heat Control 12: Stirrer
Uploaded by
Subhash DhungelCopyright:
Available Formats
Distillation
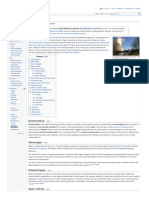
Laboratory display of distillation: 1: A heating device 2: Still pot 3: Still head 4: Thermometer/Boiling point temperature 5: Condenser 6: Cooling water in 7: Cooling water out 8: Distillate/receiving flask 9: Vacuum/gas inlet 10: Still receiver 11: Heat control 12: Stirrer speed control 13: Stirrer/heat plate 14: Heating (Oil/sand) bath 15: Stirring means e.g.(shown), boiling chips or mechanical stirrer 16: Cooling bath.[1] Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction. Commercially, distillation has a number of applications. It is used to separate crude oil into more fractions for specific uses such as transport, power generation and heating. Water is distilled to remove impurities, such as salt from seawater. Air is distilled to separate its components notably oxygen, nitrogen, and argon for industrial use. Distillation of fermented solutions has been used since ancient times to produce distilled beverages with a higher alcohol content. The premises where distillation is carried out, especially distillation of alcohol, are known as a distillery.
You might also like
- Transformation of SentencesDocument11 pagesTransformation of SentencesSubhash Dhungel100% (8)
- Ethylene UnitsDocument20 pagesEthylene UnitsLindah Turson100% (3)
- Ice Making Plant Visit ReportDocument8 pagesIce Making Plant Visit ReportAnis Badshah33% (3)
- Dry Distillation: Jump To Navigation Jump To SearchDocument2 pagesDry Distillation: Jump To Navigation Jump To SearchMohammad Jahid AlamNo ratings yet
- Distillation - WikipediaDocument16 pagesDistillation - WikipediaAbhijith B ABNo ratings yet
- API - Intro To Oil and Gas ProductionDocument10 pagesAPI - Intro To Oil and Gas Productionfoobar2016No ratings yet
- Project (Unit 1) Heriberto Jasso Pérez: "Distillation Process"Document9 pagesProject (Unit 1) Heriberto Jasso Pérez: "Distillation Process"fridda ovalleNo ratings yet
- Distillation in Chemical ProccesDocument2 pagesDistillation in Chemical ProccesFebrian Rifkhi FahrizalNo ratings yet
- ME-803 RAC Refrigeration and Air CondtitioningDocument29 pagesME-803 RAC Refrigeration and Air CondtitioningdeepaknayanNo ratings yet
- Operation Distilation Tools OkeDocument75 pagesOperation Distilation Tools Okeali budiantoNo ratings yet
- CHE 586 Lecture 2Document18 pagesCHE 586 Lecture 2Charles ObiefunaNo ratings yet
- Spray Dryer:: Pharmaceuticals Ethanol NitrogenDocument3 pagesSpray Dryer:: Pharmaceuticals Ethanol NitrogenAtal Khan PushtoonNo ratings yet
- Simple and Fractional DistillationDocument17 pagesSimple and Fractional DistillationLawand RawfNo ratings yet
- DistilationDocument11 pagesDistilationMuhammad HusseinNo ratings yet
- Whats Up!!! With The Refrigerator and Compressors - 5: Anand Plappally IIT JodhpurDocument9 pagesWhats Up!!! With The Refrigerator and Compressors - 5: Anand Plappally IIT JodhpurAyush BhadauriaNo ratings yet
- Zeolite Water Adsorption Refrigeration SystemDocument4 pagesZeolite Water Adsorption Refrigeration Systemvaibhav100% (1)
- 5.3 Experimental MethodologyDocument9 pages5.3 Experimental MethodologySiva Prasad IngetiNo ratings yet
- Crude Distillation PosterDocument1 pageCrude Distillation Postervivek_rec100% (1)
- Liquid Surface Boiling Oil Crude Oil Aristotle Evaporation Pliny The Elder CondensationDocument2 pagesLiquid Surface Boiling Oil Crude Oil Aristotle Evaporation Pliny The Elder CondensationM AhmadNo ratings yet
- Application Typical Working Fluid Specific ExampleDocument13 pagesApplication Typical Working Fluid Specific ExampleChristen RomeroNo ratings yet
- Refinery 3Document53 pagesRefinery 3Patel AshokNo ratings yet
- History: Government Polytechnic Jalna Atmospheric DistillationDocument11 pagesHistory: Government Polytechnic Jalna Atmospheric DistillationMs. YMPNo ratings yet
- Reporte 1 Evaporador FinalDocument25 pagesReporte 1 Evaporador FinalZucely CastilloNo ratings yet
- Absorption RefrigeratorDocument5 pagesAbsorption RefrigeratorThanveer Ahmed SNo ratings yet
- Steam Jet Refrigeration Cycle: Lecture - A, Week 4 Resource Person: Adeel QadirDocument11 pagesSteam Jet Refrigeration Cycle: Lecture - A, Week 4 Resource Person: Adeel QadirAbdul Hannan SandhuNo ratings yet
- Crude Oil DistillationDocument15 pagesCrude Oil DistillationAjayMehraNo ratings yet
- 1.1 Distillation and Its BackgroundDocument32 pages1.1 Distillation and Its BackgroundAhmed ElsakaanNo ratings yet
- EvaporatorsDocument1 pageEvaporatorsvijay kumar honnaliNo ratings yet
- Laboratory IceDocument7 pagesLaboratory IceFamero Franz AikenNo ratings yet
- J Jaap 2016 11 020Document40 pagesJ Jaap 2016 11 020Manicks VelanNo ratings yet
- DistillationDocument16 pagesDistillationAnonymous FGzDAs0SoNo ratings yet
- Water Cooling: NomenclatureDocument5 pagesWater Cooling: Nomenclaturemhd_bashiriNo ratings yet
- Separation Techniques: Composed With Epsilon Notes in AndroidDocument7 pagesSeparation Techniques: Composed With Epsilon Notes in AndroidBrãñdøn DzîñgáíNo ratings yet
- GEA P03e Evaporation Technology 1Document24 pagesGEA P03e Evaporation Technology 1Seyit Avcu100% (2)
- DistillationDocument5 pagesDistillationMa. Lilian Jem MonteroNo ratings yet
- IN196 03 Control SystemsDocument31 pagesIN196 03 Control SystemsEle B Ac0% (1)
- Freeze Concentration 1Document13 pagesFreeze Concentration 1A PNo ratings yet
- The Vapor Absorption Refrigeration System Consists ofDocument2 pagesThe Vapor Absorption Refrigeration System Consists ofJera ObsinaNo ratings yet
- Absorption Chiller Rev3 (MSB) 20-9Document96 pagesAbsorption Chiller Rev3 (MSB) 20-9norshakeela100% (3)
- Mam II Unit 1 (Part 2)Document18 pagesMam II Unit 1 (Part 2)Mainak PaulNo ratings yet
- Absorption: Important PetroleumDocument27 pagesAbsorption: Important PetroleumRana Muhammad Bakhtaj AyazNo ratings yet
- Natural Gas ProcessingDocument4 pagesNatural Gas ProcessingMohamad.NahhasNo ratings yet
- Literatur Modul 5 - Kesetimbangan FasaDocument18 pagesLiteratur Modul 5 - Kesetimbangan Fasasherilyn pagarintanNo ratings yet
- A Systemic Optimization Approach For The Design of Natural Gas Dehydration PlantDocument9 pagesA Systemic Optimization Approach For The Design of Natural Gas Dehydration PlantInternational Journal of Research in Engineering and ScienceNo ratings yet
- Refining Process (ARCHANA COMPLETE REFINING STUFF)Document96 pagesRefining Process (ARCHANA COMPLETE REFINING STUFF)Mahesh sinhaNo ratings yet
- Refining Process (ARCHANA COMPLETE REFINING STUFF)Document96 pagesRefining Process (ARCHANA COMPLETE REFINING STUFF)Mahesh sinhaNo ratings yet
- Yu Ki Ko KoDocument54 pagesYu Ki Ko KoThaungShanHtet PalatarNo ratings yet
- FST-404 Food Process Engineering Operation of Refrigeration Equipment Lecture No.5Document14 pagesFST-404 Food Process Engineering Operation of Refrigeration Equipment Lecture No.5Muhammad WaseemNo ratings yet
- Fresh Water GeneratorsDocument13 pagesFresh Water GeneratorsLeonardo AraujoNo ratings yet
- What Makes A Good Refrigerant?: GeneralDocument1 pageWhat Makes A Good Refrigerant?: GeneralkprasannanNo ratings yet
- Equipment and System Dehydrating, Charging, and Testing: Related Commercial ResourcesDocument7 pagesEquipment and System Dehydrating, Charging, and Testing: Related Commercial ResourcesBurning TrainNo ratings yet
- Refrigeration Efficiency - U5: Keep Your Cool - Keep Your CashDocument5 pagesRefrigeration Efficiency - U5: Keep Your Cool - Keep Your Cashbookslover1No ratings yet
- Oil Refinery - Energy EducationDocument3 pagesOil Refinery - Energy Educationmbw000012378No ratings yet
- Petroleum Refineries Waste Water - AssignmentDocument29 pagesPetroleum Refineries Waste Water - AssignmentIastra100% (2)
- Ch8 Refinery ProcessesDocument46 pagesCh8 Refinery Processesفرح100% (1)
- One Kind of Evaporator Is A Kind of Radiator Coil Used in A Closed Compressor Driven Circulation of A Liquid CoolantDocument1 pageOne Kind of Evaporator Is A Kind of Radiator Coil Used in A Closed Compressor Driven Circulation of A Liquid CoolantfikaduNo ratings yet
- Contemporary Anaesthetic Equipments.: An Aid for Healthcare ProfessionalsFrom EverandContemporary Anaesthetic Equipments.: An Aid for Healthcare ProfessionalsNo ratings yet
- Process Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersFrom EverandProcess Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersNo ratings yet
- Mechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesFrom EverandMechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesNo ratings yet
- AnalysisDocument4 pagesAnalysisSubhash DhungelNo ratings yet
- LiquidDocument7 pagesLiquidSubhash DhungelNo ratings yet
- Superposition of WavesDocument3 pagesSuperposition of WavesSubhash DhungelNo ratings yet
- Experiments On Gramimetry and Precipitation TitrationDocument4 pagesExperiments On Gramimetry and Precipitation TitrationSubhash DhungelNo ratings yet
- Assay of Acetyl Salicylic Acid and Limit of Salicylic Acid.: Title:Quality Assurance of Aspirin Tablets On The Basis ofDocument4 pagesAssay of Acetyl Salicylic Acid and Limit of Salicylic Acid.: Title:Quality Assurance of Aspirin Tablets On The Basis ofSubhash DhungelNo ratings yet
- Biological AssaysDocument19 pagesBiological AssaysSubhash DhungelNo ratings yet
- How To Do A Patient Follow-UpDocument3 pagesHow To Do A Patient Follow-UpSubhash DhungelNo ratings yet
- Absorption Methods: I) Transmittance (T)Document2 pagesAbsorption Methods: I) Transmittance (T)Subhash DhungelNo ratings yet
- Quantum Mechanical ConceptDocument4 pagesQuantum Mechanical ConceptSubhash DhungelNo ratings yet
- Mathematical Description of A WaveDocument3 pagesMathematical Description of A WaveSubhash DhungelNo ratings yet
- Quantum Mechanical ConceptDocument4 pagesQuantum Mechanical ConceptSubhash DhungelNo ratings yet
- ErrorsDocument6 pagesErrorsSubhash DhungelNo ratings yet
- Laser Sources:: ComponentsDocument5 pagesLaser Sources:: ComponentsSubhash DhungelNo ratings yet
- Sources of Coherent RadiationDocument7 pagesSources of Coherent RadiationSubhash DhungelNo ratings yet
- Energy States of Chemical Species: Two Important Postulates of Quantum TheoryDocument2 pagesEnergy States of Chemical Species: Two Important Postulates of Quantum TheorySubhash DhungelNo ratings yet
- Diffraction of RadiationDocument3 pagesDiffraction of RadiationSubhash DhungelNo ratings yet
- Analytical MethodsDocument16 pagesAnalytical MethodsSubhash Dhungel100% (1)