Professional Documents
Culture Documents
Formulario 2
Formulario 2
Uploaded by
J. Jorge Torres0 ratings0% found this document useful (0 votes)
4 views1 page1) The document contains equations for calculating thermodynamic properties such as internal energy, heat capacity at constant volume, heat capacity at constant pressure, and equations of state for ideal gases.
2) Key equations include those relating pressure, volume, and temperature for ideal gases as well as equations integrating heat capacity over temperature ranges to calculate changes in enthalpy and internal energy.
3) Properties such as heat capacity are also related based on the specific heat at constant volume and pressure for ideal gases.
Original Description:
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1) The document contains equations for calculating thermodynamic properties such as internal energy, heat capacity at constant volume, heat capacity at constant pressure, and equations of state for ideal gases.
2) Key equations include those relating pressure, volume, and temperature for ideal gases as well as equations integrating heat capacity over temperature ranges to calculate changes in enthalpy and internal energy.
3) Properties such as heat capacity are also related based on the specific heat at constant volume and pressure for ideal gases.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
4 views1 pageFormulario 2
Formulario 2
Uploaded by
J. Jorge Torres1) The document contains equations for calculating thermodynamic properties such as internal energy, heat capacity at constant volume, heat capacity at constant pressure, and equations of state for ideal gases.
2) Key equations include those relating pressure, volume, and temperature for ideal gases as well as equations integrating heat capacity over temperature ranges to calculate changes in enthalpy and internal energy.
3) Properties such as heat capacity are also related based on the specific heat at constant volume and pressure for ideal gases.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
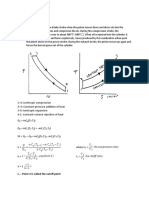
FORMULARIOSEGUNDOEXAMENPARCIAL
c =
u T
p dV =
p2V2 p1V1 1 n V2 V1
(n 1)
cp =
h T p cp c
p dV = p1V1 ln
(n = 1)
k=
T2 P2 = T1 1 P
( n 1)
n
V1 = V 2
n 1
(ideal gas )
h(T , p) h f (T ) + f (T )[ p psat (T )]
p Z= RT
pR = p pc and TR = T Tc
p dV =
mR(T2 T1 ) 1 n V2 V1
(ideal gas, n 1)
p dV = mRT ln
(ideal gas, n = 1)
c p (T ) = c (T ) + R c p (T ) = kR k 1 R k 1 c (T ) dT T2 T1
T2
& = Vn dA m
A
c (T ) =
(ideal gas )
T2
T1
cp
T1
c p (T ) dT T2 T1
You might also like
- 4862 Solutionsxin PDFDocument37 pages4862 Solutionsxin PDFይቴ ስንሻዉNo ratings yet
- French Prepositions - Les PrépositionsDocument1 pageFrench Prepositions - Les PrépositionsJ. Jorge Torres100% (1)
- Assignment 1 - Define A Project - AnsweredDocument5 pagesAssignment 1 - Define A Project - AnsweredJ. Jorge TorresNo ratings yet
- AC E-House Leaflet enDocument2 pagesAC E-House Leaflet enayah_ainaNo ratings yet
- Extra Information For Chem 340 Exams:: Transport Temperature (T), Activity/concentration (A) W RT JDocument4 pagesExtra Information For Chem 340 Exams:: Transport Temperature (T), Activity/concentration (A) W RT JZevano C. SibaraniNo ratings yet
- Formulario: Sustancias Puras Trabajo y Calor Potencia y Flujo de CalorDocument2 pagesFormulario: Sustancias Puras Trabajo y Calor Potencia y Flujo de CalorGremar Da MataNo ratings yet
- Chapter 3 - Section B - Non-Numerical SolutionsDocument10 pagesChapter 3 - Section B - Non-Numerical SolutionsFaris NaufalNo ratings yet
- Chapter 3 - Section B - Non-Numerical SolutionsDocument12 pagesChapter 3 - Section B - Non-Numerical Solutionslight2618No ratings yet
- Quantitative Phys EquationsDocument1 pageQuantitative Phys EquationsMarcus PierroNo ratings yet
- COP COP P=P P m m p p ω= m m w=∅ p p: Mezclas gas-vaporDocument2 pagesCOP COP P=P P m m p p ω= m m w=∅ p p: Mezclas gas-vaporTania MarisolNo ratings yet
- Second Law Analysis Formula PageDocument2 pagesSecond Law Analysis Formula PageFadi W MoussaNo ratings yet
- DH Du+V DP+PDV Du+V DP H V PDocument4 pagesDH Du+V DP+PDV Du+V DP H V Pbrando1819No ratings yet
- Mit3 PDFDocument2 pagesMit3 PDFrosendo rojas barraganNo ratings yet
- Uses of Maxwell RelationsDocument17 pagesUses of Maxwell RelationsArun EbenezerNo ratings yet
- Physical Chemistry FormulasDocument1 pagePhysical Chemistry FormulasDelphinacro100% (1)
- School of Chemical Engineering: Chem 251 Assignment 1 and 2Document16 pagesSchool of Chemical Engineering: Chem 251 Assignment 1 and 2Keevani NaidooNo ratings yet
- Formulario TermodinamicaDocument1 pageFormulario TermodinamicaHerbNo ratings yet
- Paper Thermo Mechanical EngineeringDocument14 pagesPaper Thermo Mechanical EngineeringAdif HerawanNo ratings yet
- Lecture 6 - Summer 2023-24Document10 pagesLecture 6 - Summer 2023-24Mehedy Hasan RidoyNo ratings yet
- PC1 Formulaesheet FinalDocument3 pagesPC1 Formulaesheet FinalIzzat Fuad ParkjatNo ratings yet
- Formula For Engineering Thermodynamics I 254231 Semester 1 2550 Concept andDocument1 pageFormula For Engineering Thermodynamics I 254231 Semester 1 2550 Concept andKenneth MayorNo ratings yet
- Fiqui ParcialDocument7 pagesFiqui ParcialCarlosCristobalNo ratings yet
- UO2016F Slide 1 - Basic Relations and Equations of Heat ConductionDocument20 pagesUO2016F Slide 1 - Basic Relations and Equations of Heat ConductionSushil KumarNo ratings yet
- PC 1 Formulae Sheet 1Document2 pagesPC 1 Formulae Sheet 1Kaaya GodfreyNo ratings yet
- Thermodynamics FormulaeDocument2 pagesThermodynamics FormulaeHelmi Hamzah100% (3)
- Assignment FINALDocument67 pagesAssignment FINALlaila khanNo ratings yet
- Helpful Eqns ME311 F14 PJFDocument2 pagesHelpful Eqns ME311 F14 PJFtarzantrapNo ratings yet
- Ideal Gas FormulasDocument2 pagesIdeal Gas FormulasbythekiloNo ratings yet
- CIVE322 Equations 2010Document2 pagesCIVE322 Equations 2010spjoshi108No ratings yet
- Compressible Flow PDFDocument90 pagesCompressible Flow PDFOmer TokhNo ratings yet
- Departure FunctionDocument6 pagesDeparture FunctionzidinhoNo ratings yet
- P P A U M T A M P P U U: AS301, IITM, Lecture 2 (06/08/2007)Document3 pagesP P A U M T A M P P U U: AS301, IITM, Lecture 2 (06/08/2007)Sunil VermaNo ratings yet
- Che 254 (Midsem)Document6 pagesChe 254 (Midsem)obumaradonaNo ratings yet
- Formulario-2012 13Document2 pagesFormulario-2012 13inmarf5No ratings yet
- Unit One and ThreeDocument32 pagesUnit One and ThreeGAURAV RATHORENo ratings yet
- VALEM2Document1 pageVALEM2Hans PriksNo ratings yet
- TER201 Lecture 6Document66 pagesTER201 Lecture 6lnxxNo ratings yet
- 2.1.2 Stirred Tank Heater Solution:: AL DT DT F T T Qe PC Qe U A T T)Document7 pages2.1.2 Stirred Tank Heater Solution:: AL DT DT F T T Qe PC Qe U A T T)Ed Ryan SorianoNo ratings yet
- Equation Thermal Diesel CycleDocument2 pagesEquation Thermal Diesel CycleZakyKikyNo ratings yet
- Fundamentos de Aeron Autica Formulario: Instructor: Arturo Galv An Rdz. 12 de Noviembre de 2009Document3 pagesFundamentos de Aeron Autica Formulario: Instructor: Arturo Galv An Rdz. 12 de Noviembre de 2009agalvan1311No ratings yet
- Me200 - Eqnsheet 12 Jun 2012Document2 pagesMe200 - Eqnsheet 12 Jun 2012klinNo ratings yet
- Kompresible - 1Document11 pagesKompresible - 1isudirmanNo ratings yet
- Correction of Final January 2022Document3 pagesCorrection of Final January 2022s2ne228No ratings yet
- Solution: 1. Refer Eq. (6.28)Document4 pagesSolution: 1. Refer Eq. (6.28)kajal mishrsNo ratings yet
- Ideal GasDocument21 pagesIdeal GasElia AliNo ratings yet
- Formula Sheet Midterm 1Document1 pageFormula Sheet Midterm 1qwqeNo ratings yet
- UntitledDocument2 pagesUntitledAlireza Pir-MohamadiNo ratings yet
- Compressible Flow PDFDocument210 pagesCompressible Flow PDFRicky JuwonoNo ratings yet
- Only 1 PageDocument2 pagesOnly 1 PageAbhijit RoyNo ratings yet
- TD MODULE 4Document12 pagesTD MODULE 4mujeebNo ratings yet
- FORMULARIO 2012icDocument2 pagesFORMULARIO 2012icWildor Cordova SanchezNo ratings yet
- 1001 Formula Sheet - PDF 3Document3 pages1001 Formula Sheet - PDF 3Hafizudin HafizNo ratings yet
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument7 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- Adiabatic ChangeDocument2 pagesAdiabatic ChangeYau Ching KoonNo ratings yet
- Task 1Document9 pagesTask 1Julius CagampangNo ratings yet
- Kmme Te101-3Document6 pagesKmme Te101-3ayla sözenNo ratings yet
- Formula RioDocument2 pagesFormula RioOswaldo LinaresNo ratings yet
- Equation 2Document7 pagesEquation 2Oka29No ratings yet
- PHYS 310: Thermodynamics and Statistical Mechanics Final Exam Formula SheetDocument2 pagesPHYS 310: Thermodynamics and Statistical Mechanics Final Exam Formula SheetChristopher ThaiNo ratings yet
- Termodinamica ProblemDocument6 pagesTermodinamica ProblemJuandearco JuanarcoNo ratings yet
- FoundationsDocument1 pageFoundations이소연[ 학부재학 / 화공생명공학과 ]No ratings yet
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- Innovative PlanningDocument1 pageInnovative PlanningJ. Jorge TorresNo ratings yet
- Leadership Development PathDocument2 pagesLeadership Development PathJ. Jorge TorresNo ratings yet
- Outstanding Toastmaster Guidelines - Jorge TorresDocument2 pagesOutstanding Toastmaster Guidelines - Jorge TorresJ. Jorge TorresNo ratings yet
- Shimano BR-MT410 User Manual (English - 1 Pages)Document3 pagesShimano BR-MT410 User Manual (English - 1 Pages)J. Jorge TorresNo ratings yet
- Ehouse 2Document1 pageEhouse 2J. Jorge TorresNo ratings yet
- Aggregation StrategiesFeb2012 PDFDocument6 pagesAggregation StrategiesFeb2012 PDFJ. Jorge TorresNo ratings yet
- Brochure eHouse-SiemensDocument22 pagesBrochure eHouse-SiemensJ. Jorge TorresNo ratings yet
- SOFEC Corporate BrochureDocument20 pagesSOFEC Corporate BrochureJ. Jorge TorresNo ratings yet
- SOFEC CALM BUOY FINAL 3page RedDocument3 pagesSOFEC CALM BUOY FINAL 3page RedJ. Jorge TorresNo ratings yet
- List of Standards For Elecrical Equipment - App2 PDFDocument16 pagesList of Standards For Elecrical Equipment - App2 PDFJ. Jorge Torres100% (1)
- How Uber Uses Psychological Tricks To Push Its Drivers' Buttons - Alaska Dispatch News PDFDocument5 pagesHow Uber Uses Psychological Tricks To Push Its Drivers' Buttons - Alaska Dispatch News PDFJ. Jorge TorresNo ratings yet
- Brochure eHouse-Eaton (IPA)Document6 pagesBrochure eHouse-Eaton (IPA)J. Jorge TorresNo ratings yet
- 5.3.2 Loops: 5.3.3 Conditionals 5.3 Assembly Language Examples 5.3.1 VariablesDocument3 pages5.3.2 Loops: 5.3.3 Conditionals 5.3 Assembly Language Examples 5.3.1 VariablesJ. Jorge TorresNo ratings yet