Professional Documents
Culture Documents
The Science/Mechanics of Breathing
The Science/Mechanics of Breathing
Uploaded by
Van MichaelOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
The Science/Mechanics of Breathing
The Science/Mechanics of Breathing
Uploaded by
Van MichaelCopyright:
Available Formats
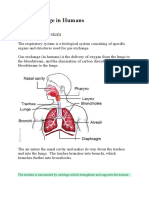
The Science/Mechanics Of Breathing
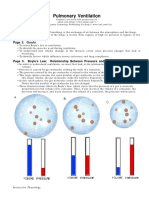
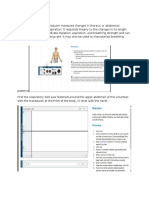
Boyles Law: The pressure of a given quantity of gas is inversely proportional to its volume. Increasing volume results in decreasing pressure. Decreasing volume results in Increasing pressure. Increasing volume causes a decrease in pressure which causes air to rush in as the pressures reached equilibrium. Decreasing volume causes an increase in pressure which causes air to expelled as pressures reached equilibrium.
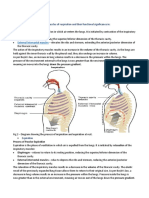
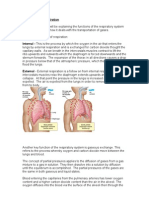
The mechanics of breathing involve changing the volume and pressure of the thoracic cavity. By using the principles of Boils law, one can see that the pressure in the thoracic cavity is inversely proportional to its volume. When the intercostals muscles contract the ribs are elevated. At the same time the diaphragm contracts. These events expand the thoracic cavity, decreasing its internal pressure. The lungs expand, filling the thoracic cavity. The resulting pressure in the lungs is lower than that outside the body. Air enters the lungs until equilibrium is reached. When the diaphragm and the intercostals muscles relax the thoracic cavity recoils. The resulting increase in pressure cause the air within the lungs to be expelled.
You might also like
- Physiology, Boyle's Law - StatPearls - NCBI BookshelfDocument3 pagesPhysiology, Boyle's Law - StatPearls - NCBI BookshelfChris IidaNo ratings yet
- Mechanism of Breathing: Oleh: Dhyani Chitta MayasariDocument13 pagesMechanism of Breathing: Oleh: Dhyani Chitta MayasariDhyani Chitta MayasariNo ratings yet
- Respiratory System-1Document84 pagesRespiratory System-1pranjal raiNo ratings yet
- Study Guide 4: Diaphragm External Intercostal MusclesDocument37 pagesStudy Guide 4: Diaphragm External Intercostal MusclesItadori YujiNo ratings yet
- Definition of Boyle's Law: What Is An Ideal Gas?Document4 pagesDefinition of Boyle's Law: What Is An Ideal Gas?حسين كاظم ياسينNo ratings yet
- Respiratory System 2Document48 pagesRespiratory System 2Sumita GhoshNo ratings yet
- Physiology of RespirationDocument2 pagesPhysiology of RespirationIOSRjournalNo ratings yet
- The Thoracic Cavity: Atmospheric Pressure Intrapulmonary PressureDocument4 pagesThe Thoracic Cavity: Atmospheric Pressure Intrapulmonary PressureNylaxor Erbmeicet OicacaNo ratings yet
- Assignment Respiratory During ExerciseDocument3 pagesAssignment Respiratory During Exercisedomhughes1093No ratings yet
- Gas Exchange in HumansDocument5 pagesGas Exchange in HumansRAGHU RAM SAKAMURINo ratings yet
- Mechanical VentilationDocument262 pagesMechanical VentilationShalini Garg0% (1)
- PHYSIOLOGYDocument9 pagesPHYSIOLOGYS GNo ratings yet
- Physiology of Respiration: Course Code: BBS01T1008 Course Name: Biology For EngineersDocument15 pagesPhysiology of Respiration: Course Code: BBS01T1008 Course Name: Biology For EngineersDivya TripathyNo ratings yet
- The Effect of Exercise On The Cardiorespiratory SystemDocument15 pagesThe Effect of Exercise On The Cardiorespiratory SystemBASAVARAJ100% (1)
- BreathingDocument14 pagesBreathingHasan KontyNo ratings yet
- LoveubabykoDocument2 pagesLoveubabykoSheila Mae NimNo ratings yet
- Ventilation and Respiratory VolumesDocument10 pagesVentilation and Respiratory Volumesalthea jade villadongaNo ratings yet
- Lecture Notes On Respiratory Physiology PDFDocument33 pagesLecture Notes On Respiratory Physiology PDFMiles HuiNo ratings yet
- Human Gas ExchangeDocument29 pagesHuman Gas Exchangeuinatohaturn78No ratings yet
- RespirationDocument7 pagesRespirationMemona KhalidNo ratings yet
- Respiratory System Structure and FunctionDocument6 pagesRespiratory System Structure and FunctionRavi BhollahNo ratings yet
- 11 Lung PhysiologyDocument14 pages11 Lung PhysiologyGlen UrbaniNo ratings yet
- What Is Gas ExchangeDocument1 pageWhat Is Gas ExchangeTajwar TaqiNo ratings yet
- Physio. Lec.2 Pulmonary VentilationDocument18 pagesPhysio. Lec.2 Pulmonary Ventilationزينب فؤاد موسىNo ratings yet
- Process of BreathingDocument3 pagesProcess of BreathingssmaddiNo ratings yet
- Mechanism of BreathingDocument1 pageMechanism of BreathingjaysraelNo ratings yet
- Respiratory SystemDocument6 pagesRespiratory SystemAthena CrooksNo ratings yet
- Pulmonary Ventilation PDFDocument11 pagesPulmonary Ventilation PDFSrsblackieNo ratings yet
- Respiratory Physiology: Part IbDocument40 pagesRespiratory Physiology: Part IbKristian CadaNo ratings yet
- Respiratory System OkDocument36 pagesRespiratory System Okakselerasi10No ratings yet
- Function of The Respiratory SystemDocument5 pagesFunction of The Respiratory SystemRachelHatcher123No ratings yet
- Evens RespiortyDocument4 pagesEvens RespiortyamyhowarthNo ratings yet
- Gas Exchange in HumansDocument4 pagesGas Exchange in HumansAky IwnlNo ratings yet
- The Respiratory SystemDocument2 pagesThe Respiratory SystemconnievelardeNo ratings yet
- G10 Science Q4 - Boyle's Law (Gas Law)Document13 pagesG10 Science Q4 - Boyle's Law (Gas Law)Sky HadesNo ratings yet
- Features of Gas Exchange Surfaces: The Alveolus Is The Gas Exchange Surface in HumansDocument9 pagesFeatures of Gas Exchange Surfaces: The Alveolus Is The Gas Exchange Surface in HumansM. ShayanNo ratings yet
- 4.2 - Respiratory SystemDocument10 pages4.2 - Respiratory SystemBenJenkinsNo ratings yet
- Pulmonary VentilationDocument24 pagesPulmonary VentilationAreeba KhanNo ratings yet
- Breathing: Breathing Is The Process That Moves Air in and Out of The LungsDocument5 pagesBreathing: Breathing Is The Process That Moves Air in and Out of The LungsArief Rachman WiladisastraNo ratings yet
- BreathingDocument5 pagesBreathingGelyn Lanuza-AsuncionNo ratings yet
- 16 Pulmonary Ventsilation CH16 2Document34 pages16 Pulmonary Ventsilation CH16 2Brodsky MacedoNo ratings yet
- 24 - Physiology of The Respiratory System PDFDocument38 pages24 - Physiology of The Respiratory System PDFlovelyc95No ratings yet
- Mechanism of BreathingDocument2 pagesMechanism of BreathingMonikita PerezNo ratings yet
- The Respiratory SystemDocument2 pagesThe Respiratory SystemRaluca MoldovanNo ratings yet
- المحاضرة - الثالثة الجاهزة - فسيو - بلك - التنفسي - دفعة - خامسة -Document26 pagesالمحاضرة - الثالثة الجاهزة - فسيو - بلك - التنفسي - دفعة - خامسة -bessan alfqeatNo ratings yet
- Forum Fisiologi SenamDocument3 pagesForum Fisiologi SenamErwan Sha HabinullahNo ratings yet
- Document 5Document5 pagesDocument 5kauthar hassanNo ratings yet
- University of Guyana School of Medicine Med 1106 - Physiology I DR Kalima ThompsonDocument66 pagesUniversity of Guyana School of Medicine Med 1106 - Physiology I DR Kalima ThompsonKNo ratings yet
- Lab Exercise 9 Respiratory System PhysiologyDocument5 pagesLab Exercise 9 Respiratory System PhysiologySophia Nicole RosalesNo ratings yet
- Physiology of Respiratory SystemDocument9 pagesPhysiology of Respiratory SystemAngelica Joan SorianoNo ratings yet
- RespirationDocument12 pagesRespirationjimmycrackle710No ratings yet
- Microsoft Word - Altitude PhysiologyDocument49 pagesMicrosoft Word - Altitude PhysiologyMetinee AngsutorntakNo ratings yet
- 11.1 The Breathing System: Features of Gas Exchange SurfacesDocument13 pages11.1 The Breathing System: Features of Gas Exchange SurfacesX GamerNo ratings yet
- Unit 2 - Respiratory Physiology HandoutDocument7 pagesUnit 2 - Respiratory Physiology Handouttilahun aligazNo ratings yet
- Respiratory System: Departement of Physiology Medical Faculty of Universitas Sumatera UtaraDocument142 pagesRespiratory System: Departement of Physiology Medical Faculty of Universitas Sumatera Utara292Vien HardiyantiNo ratings yet
- Respiration Lecture NotesDocument7 pagesRespiration Lecture Notesamrutha mohandasNo ratings yet
- VentilationDocument16 pagesVentilationsamar yousif mohamedNo ratings yet
- THE BUTEYKO METHOD (Translated): The secret of controlled breathing for health, well-being and vitalityFrom EverandTHE BUTEYKO METHOD (Translated): The secret of controlled breathing for health, well-being and vitalityNo ratings yet
- The Basic Breathwork Book: A Fundamental Guide to Enhancing Health, Performance and MindfulnessFrom EverandThe Basic Breathwork Book: A Fundamental Guide to Enhancing Health, Performance and MindfulnessNo ratings yet
- E ReceiptDocument1 pageE ReceiptVan MichaelNo ratings yet
- Michaelvanddivinagracia : Bombardiertransportation15F Pgutowerasiatownitpark 6014apaslahugcebucityDocument4 pagesMichaelvanddivinagracia : Bombardiertransportation15F Pgutowerasiatownitpark 6014apaslahugcebucityVan MichaelNo ratings yet
- Scholarship Application FormDocument6 pagesScholarship Application FormVan MichaelNo ratings yet
- A. Demand A.1. Past DemandDocument3 pagesA. Demand A.1. Past DemandVan MichaelNo ratings yet
- History of Lean ManufacturingDocument14 pagesHistory of Lean ManufacturingVan Michael100% (2)
- Letter To Matea ClimbingDocument1 pageLetter To Matea ClimbingVan MichaelNo ratings yet
- My Accomplishment Report VGDocument32 pagesMy Accomplishment Report VGVan Michael100% (2)