Professional Documents
Culture Documents
Salt Analysis Cheat Sheet
Salt Analysis Cheat Sheet
Uploaded by
Dev BeckhamOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Salt Analysis Cheat Sheet
Salt Analysis Cheat Sheet
Uploaded by
Dev BeckhamCopyright:
Available Formats
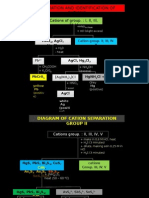
Cations Group Group Reagent Radicals Zero Group O.S. + NaOH + Heat NH4+ I Group O.S.
+ HCl Pb2+ II Group O.S. + HCI + H2S Cu2+ III Group O.S. +NH4Cl + NH4OH AI2+ ,Fe3+ IV Group O.S. + NH4CI + NH4OH +H2S Ni2+ ,Mn2+ , Zn2+ ,Co2+ V Group O.S. + NH4CI + NH4OH + (NH4)2CO3 Ba2+, Ca3+, Sr2+ VI Group O.S + NH4CI + NH4OH + NaH2PO4 Mg2+ Anions Group Group Reagent Radicals I Group Salt + dil. H2SO4 CO32-, SO32-, S2-, NO2II Group Salt + conc. H2SO4 Cl-, Br-, I-, CH3COOAdd Cu piece and heat NOAdd KMnO 4 soln. c2o42III Group O.S. + BaC!2 so42IV Group O.S + Conc.HNO3 + Heat + (NH4)24 MoO PO43Preliminary Tests Test Observation Inference Physical Appearance Colour White NH4+, Pb2+, Al3+ , Zn2+, Ba2+, Ca2+ , Sr2+ and Mg2+ Blue Cu2+ Green Ni2+ Brown Fe3+ Red Co2+ Light pink Mn2+ Odour Smell of NH3 NH4+ Smell of vinegar CH3COOSmell of rotten eggs H2Dry Heating Colourless gas evolved CO32- , SO32-, S2- and ClBlown gas evolved Br -, NO3Violet gas evolved ICrackling sound Pb(NO3)2 and Ba(NO3)2 Brown residue Cu2+ Flame Test Greenish blue Cu2+ Light green Ba2+ Brick red Ca2+ Crimson red Sr2+ Know more about: Simultaneous Cation and Anion Analysis with Charged
You might also like
- Chemical Formula Writing WorksheetDocument4 pagesChemical Formula Writing Worksheetprabhu4321100% (1)
- (Study Material) Salt Analysis of Cations and Anions (Chemistry) - CBSE PORTAL - CBSE, ICSE, NIOS, JEE-MAIN, AIPMT Students CommunityDocument3 pages(Study Material) Salt Analysis of Cations and Anions (Chemistry) - CBSE PORTAL - CBSE, ICSE, NIOS, JEE-MAIN, AIPMT Students CommunityRaiNo ratings yet
- SaltDocument2 pagesSaltRonit SahaNo ratings yet
- Salt AnalysisDocument2 pagesSalt AnalysisfundocfunnyNo ratings yet
- Salt AnalysisDocument2 pagesSalt AnalysissuryavignesNo ratings yet
- Separation of CationsDocument9 pagesSeparation of CationsQodri Maulana SipahutarNo ratings yet
- Salt Analysis: CationsDocument2 pagesSalt Analysis: CationsAkshyansh KumarNo ratings yet
- Chemical ReactionsDocument6 pagesChemical ReactionsKushNo ratings yet
- CationsDocument2 pagesCationsSyam SankarNo ratings yet
- Salt Analysis Class 11Document2 pagesSalt Analysis Class 11Siddharth Roy100% (1)
- Sblock RXDocument2 pagesSblock RXniranjanyadawad14No ratings yet
- WS BalancingDocument2 pagesWS Balancingapi-3706290100% (1)
- Salt AnalysisDocument4 pagesSalt AnalysisNimay RastogiNo ratings yet
- Anions Group 1: (Salt + Dil. H2SO4)Document4 pagesAnions Group 1: (Salt + Dil. H2SO4)UshasreeSanyalNo ratings yet
- Salt Analysis Class 11 &12 ChemistryDocument4 pagesSalt Analysis Class 11 &12 Chemistryritikbhatia530No ratings yet
- Worksheet G10 InorganicEqn 1Document2 pagesWorksheet G10 InorganicEqn 1SantanuNo ratings yet
- Balancing Equations WsDocument2 pagesBalancing Equations WsPranay KhannaNo ratings yet
- Arrangement of Coefficients in The Equations of Redox ReactionsDocument11 pagesArrangement of Coefficients in The Equations of Redox Reactionslorenlori2004No ratings yet
- UntitledDocument2 pagesUntitledapi-233404189No ratings yet
- Untitled Spreadsheet - Google SheetsDocument3 pagesUntitled Spreadsheet - Google SheetsVIV GamingNo ratings yet
- Activity 8 NomenclatureDocument2 pagesActivity 8 NomenclatureCyruss MeranoNo ratings yet
- Equations To Be BalancedDocument10 pagesEquations To Be BalancedDesmond Dujon HenryNo ratings yet
- Week 5 - QualitativeDocument26 pagesWeek 5 - QualitativeMa. Nathalie Claire G. GadiaNo ratings yet
- (Study Material) Salt Analysis of Cations and Anions (Chemistry) - CBSE PORTAL - CBSE, ICSE, NIOS, JEE-MAIN, AIPMT Students CommunityDocument4 pages(Study Material) Salt Analysis of Cations and Anions (Chemistry) - CBSE PORTAL - CBSE, ICSE, NIOS, JEE-MAIN, AIPMT Students Communityganesh kumarNo ratings yet
- 06 Salt-02Document2 pages06 Salt-0211thA -13-DarshilNo ratings yet
- Balance ArDocument2 pagesBalance ArIvan Dario Pineda PatiñoNo ratings yet
- Nitric AcidDocument27 pagesNitric Acidaanika5411No ratings yet
- Basic Inorganic Chemistry PHR 125: Prof. Dr. Mona BedairDocument33 pagesBasic Inorganic Chemistry PHR 125: Prof. Dr. Mona BedairAvvari AnnamaniNo ratings yet
- Exer Ajust Reac TGLDocument2 pagesExer Ajust Reac TGLPaula CortesNo ratings yet
- Redox ReactionDocument3 pagesRedox ReactionPranjal GuptaNo ratings yet
- Analitycal Chemistry - Lecture 06Document38 pagesAnalitycal Chemistry - Lecture 06Irene BoyaNo ratings yet
- Balancing EqueationsDocument6 pagesBalancing EqueationsSurendra ZirpeNo ratings yet
- Oxidation NumberDocument14 pagesOxidation Numbermysha moontahaNo ratings yet
- Chemistry, C8A - Aanotes (S)Document26 pagesChemistry, C8A - Aanotes (S)Farah Aisyah AhmadNo ratings yet
- Zapamti - Kiseline, Lu Ine, SoliDocument2 pagesZapamti - Kiseline, Lu Ine, SolidragoNo ratings yet
- P-Block Board Youtube Part-2Document53 pagesP-Block Board Youtube Part-2vaibhav sainiNo ratings yet
- Lecture 1 EquationsDocument11 pagesLecture 1 Equationsmerabamoding11No ratings yet
- PDF Created With Pdffactory Pro Trial VersionDocument5 pagesPDF Created With Pdffactory Pro Trial Versionvictor555555100No ratings yet
- Red Ox BalancingDocument2 pagesRed Ox BalancingLuis Panduro AlvaNo ratings yet
- Balancing Equations: Practice ProblemsDocument10 pagesBalancing Equations: Practice ProblemsSheryl Nishmae Bernardo SantosNo ratings yet
- Wan Noor Afifah BT Wan YusoffDocument33 pagesWan Noor Afifah BT Wan YusoffThilagavathyNo ratings yet
- On Tap Can Bang PTHH Lop 8Document2 pagesOn Tap Can Bang PTHH Lop 8vnmath94No ratings yet
- iNORGANIC Salt AnalysisDocument12 pagesiNORGANIC Salt AnalysisNishant KaushikNo ratings yet
- Worksheets RRDocument4 pagesWorksheets RRHrithik JerathNo ratings yet
- Red Ox BalancingDocument2 pagesRed Ox BalancingAhmed KazazNo ratings yet
- Module SaltDocument12 pagesModule SaltAzie Nurul Akhtar100% (1)
- 10.5. Chemical Nomenclature - Molecular Compounds and AcidsDocument17 pages10.5. Chemical Nomenclature - Molecular Compounds and AcidsNina Anne Marie PascualNo ratings yet
- CH 3 Chemical Reaction WorksheetDocument19 pagesCH 3 Chemical Reaction WorksheetStephanus AbednegoNo ratings yet
- Nalytical Hemistry: Identification of Acidic RadicalsDocument1 pageNalytical Hemistry: Identification of Acidic RadicalsdheerajpradeepNo ratings yet
- Assignment Neutralisation Reaction Answer KeyDocument1 pageAssignment Neutralisation Reaction Answer KeyMaridjan WiwahaNo ratings yet
- Ejercicios de Calculos QuimicosDocument70 pagesEjercicios de Calculos QuimicosMelinda AndersonNo ratings yet
- Progress in Inorganic ChemistryFrom EverandProgress in Inorganic ChemistryKenneth D. KarlinNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Practice Makes Perfect in Chemistry: Compounds, Reactions and MolesFrom EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and MolesNo ratings yet
- Practice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersFrom EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersRating: 3 out of 5 stars3/5 (2)
- Unusual Structures and Physical Properties in Organometallic ChemistryFrom EverandUnusual Structures and Physical Properties in Organometallic ChemistryNo ratings yet
- Hyrdogen Storage TechnologiesFrom EverandHyrdogen Storage TechnologiesMehmet SankirNo ratings yet