Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
33 viewsUnit 6 Review
Unit 6 Review
Uploaded by
mamazookeeprThis document provides a review of key concepts in chemistry including:
1) How to label the parts of a chemical reaction including reactants, products, and the arrow to show the reaction occurs.
2) Definitions of common chemistry terms like stoichiometry, catalysts, and reaction rate.
3) Factors that affect the rate of a chemical reaction such as concentration, temperature, and surface area.
4) How to analyze energy diagrams to determine if heat is absorbed or released by a reaction.
5) Practice balancing chemical equations and identifying the type of reaction.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You might also like
- Chapter 1 - Study GuideDocument7 pagesChapter 1 - Study Guidesarahleeabc100% (1)
- Mid Year Exam Chemistry Form 4Document13 pagesMid Year Exam Chemistry Form 4Wan Shuhaimi73% (11)
- Sample Lab ReportDocument3 pagesSample Lab Reportmamazookeepr63% (8)
- Practice Exam 2 Organic Chemistry 237 UWDocument7 pagesPractice Exam 2 Organic Chemistry 237 UWNgoc Minh NgoNo ratings yet
- Activity SheetDocument3 pagesActivity Sheetjanice alquizar100% (1)
- Experimental Design POGIL 2013Document3 pagesExperimental Design POGIL 2013Steffi Basnet0% (1)
- Enzyme Graphing Worksheet: NameDocument2 pagesEnzyme Graphing Worksheet: NamePBFNo ratings yet
- Name - Date - PeriodDocument2 pagesName - Date - PeriodmamazookeeprNo ratings yet
- BIOCATALYSISDocument4 pagesBIOCATALYSISanis azizNo ratings yet
- Types of Chemical RX WSHDocument1 pageTypes of Chemical RX WSHLeonardo SierraNo ratings yet
- Eche0807 at C1Document6 pagesEche0807 at C1Hema LataNo ratings yet
- Chemical ReactionDocument4 pagesChemical ReactionABDULRAHMAN ABDRABBONo ratings yet
- 2022 Chemistry Paper I (Theory) MJ CLUSTERDocument11 pages2022 Chemistry Paper I (Theory) MJ CLUSTERishmaeljonas681No ratings yet
- Activation EnergyDocument13 pagesActivation EnergydrtanilbabuNo ratings yet
- Chemistry Mcse PiDocument10 pagesChemistry Mcse PiMoses Samalani100% (1)
- RateofReactionsQuiz 1Document1 pageRateofReactionsQuiz 1Mulki MohamedNo ratings yet
- Cellular Respiration QuestionsDocument2 pagesCellular Respiration Questionstestingacc177No ratings yet
- Microsoft Word - CH 12 Worksheet 1-3 - DocDocument8 pagesMicrosoft Word - CH 12 Worksheet 1-3 - DocMichelle NgNo ratings yet
- Chemistry Unit 7 Test: - /32 Points PossibleDocument6 pagesChemistry Unit 7 Test: - /32 Points Possibleapi-516602929No ratings yet
- Year 10 Chemistry Time: 2 HoursDocument12 pagesYear 10 Chemistry Time: 2 HoursAdrianHedleyNo ratings yet
- UntitledDocument16 pagesUntitledMichel ElizeeNo ratings yet
- Organic HgcdsynthesisDocument10 pagesOrganic HgcdsynthesisTannishk MankarNo ratings yet
- 2022 DEDZA MOCK EXAMS (CHEMIS Paper 1Document10 pages2022 DEDZA MOCK EXAMS (CHEMIS Paper 1hamzahbrave28No ratings yet
- Year 10 Chemistry Time: 2 HoursDocument9 pagesYear 10 Chemistry Time: 2 HoursAdrianHedleyNo ratings yet
- Rates of Reaction - Mini LabsDocument7 pagesRates of Reaction - Mini LabsPolly LeungNo ratings yet
- Enzyme Graphing Worksheet FormativeDocument3 pagesEnzyme Graphing Worksheet FormativeVALENTINA PEÑALOZA HERRERANo ratings yet
- PSCH 0814 KeyDocument2 pagesPSCH 0814 KeyJan Ira RenolayanNo ratings yet
- 2.8.5.c WorksheetDocument2 pages2.8.5.c WorksheetAftab AhmedNo ratings yet
- Q4 W7 8 Sci10 LawDocument8 pagesQ4 W7 8 Sci10 LawBa BengNo ratings yet
- Las Co2Document4 pagesLas Co2angel pranadaNo ratings yet
- Pre-Lab Template - 230507 - 174440Document19 pagesPre-Lab Template - 230507 - 174440WAN AZALEEYA BINTI WAN AZANI / UPMNo ratings yet
- Chapter 7 Chemical ReactionsDocument10 pagesChapter 7 Chemical Reactionsapi-30718309No ratings yet
- Description Penerangan Temperature Suhu (°c)Document4 pagesDescription Penerangan Temperature Suhu (°c)Norlailatulakma BolhassanNo ratings yet
- CollisionTheorySE Revised For MYP-GizmosDocument9 pagesCollisionTheorySE Revised For MYP-GizmosNo HumorNo ratings yet
- PAT Bio f4 P2 2015Document14 pagesPAT Bio f4 P2 2015unieezzNo ratings yet
- ds24 Enzyme Review AssessmentDocument8 pagesds24 Enzyme Review Assessmentapi-110789702No ratings yet
- 6.2 and 6.3 Worksheet Factors and ExplainationDocument2 pages6.2 and 6.3 Worksheet Factors and ExplainationhairtNo ratings yet
- A-Level Biology Protein Exam QuestionsDocument27 pagesA-Level Biology Protein Exam Questionsfreyamartin90676No ratings yet
- c6 The Rate and Extent of Chemical Change HTDocument73 pagesc6 The Rate and Extent of Chemical Change HTewfjehwjfNo ratings yet
- Ujian Setara 2 Kimia Form4Document2 pagesUjian Setara 2 Kimia Form4adikmukNo ratings yet
- Final Term Practice Work Sheet 1aDocument2 pagesFinal Term Practice Work Sheet 1aKhondokar TarakkyNo ratings yet
- Student Exploration: Collision TheoryDocument7 pagesStudent Exploration: Collision TheoryNicholas CrowellNo ratings yet
- Chemistry Time Allowed: 1 Hour Paper 2 Theory Total Marks: /45Document7 pagesChemistry Time Allowed: 1 Hour Paper 2 Theory Total Marks: /45Salman Ul MoazzamNo ratings yet
- Physical and Chemical Changes WorksheetDocument4 pagesPhysical and Chemical Changes WorksheetAndrew ChenNo ratings yet
- NewDocument1 1Document18 pagesNewDocument1 1Paweł KorczakNo ratings yet
- Activity No. 2 & 3 Structure in Organic Compounds: Use of Molecular Models Report SheetDocument2 pagesActivity No. 2 & 3 Structure in Organic Compounds: Use of Molecular Models Report Sheetlara skwngrNo ratings yet
- Enzyme Work SheetDocument6 pagesEnzyme Work SheetmdonohueHGHSNo ratings yet
- Lesson 1 - Energy and States of Matter-7apDocument2 pagesLesson 1 - Energy and States of Matter-7apunicornn2990No ratings yet
- ds36 Enzyme Review Summative AssessmentDocument4 pagesds36 Enzyme Review Summative Assessmentapi-110789702No ratings yet
- Worksheet 8.3 (Ionic Equation Step by Step)Document2 pagesWorksheet 8.3 (Ionic Equation Step by Step)ChantoniNo ratings yet
- IBO 2008 Mumbai Theory Examination 2Document80 pagesIBO 2008 Mumbai Theory Examination 2Ari GaoNo ratings yet
- ch21 1readingassignmentDocument2 pagesch21 1readingassignmentapi-324905565No ratings yet
- The Periodic Table WorksheetDocument1 pageThe Periodic Table WorksheetZ KNo ratings yet
- Organic 1Document6 pagesOrganic 1Jaspar GlagovsNo ratings yet
- Workshop - Fuels-Fractional Distillation-CrackingDocument4 pagesWorkshop - Fuels-Fractional Distillation-Crackingmeganekokun kawaiiNo ratings yet
- Ict6-Worksheet-No.6 With Key AnswerDocument3 pagesIct6-Worksheet-No.6 With Key Answerjhe azulNo ratings yet
- Covalent Substances TestDocument7 pagesCovalent Substances TestJaspar GlagovsNo ratings yet
- Use Your Notes From The Atomic Structure Program To Answer The Following QuestionsDocument1 pageUse Your Notes From The Atomic Structure Program To Answer The Following QuestionsAngieOrtegaNo ratings yet
- AtomstructurewkstDocument1 pageAtomstructurewkstapi-270967967No ratings yet
- Grade 9 Technology Final ExamDocument8 pagesGrade 9 Technology Final ExamLaila KaderNo ratings yet
- Hydrate Lab: Never Place A Hot Crucible On A Balance! When Cool, Determine The Mass of The Crucible andDocument1 pageHydrate Lab: Never Place A Hot Crucible On A Balance! When Cool, Determine The Mass of The Crucible andmamazookeeprNo ratings yet
- Build A Trend LabDocument2 pagesBuild A Trend LabmamazookeeprNo ratings yet
- Chemical Formula QuizDocument3 pagesChemical Formula QuizmamazookeeprNo ratings yet
- Unit 4 Test 2009Document2 pagesUnit 4 Test 2009mamazookeeprNo ratings yet
- Subatomic Particle WorksheetDocument1 pageSubatomic Particle WorksheetmamazookeeprNo ratings yet
- Average Weighted Atomic MassDocument2 pagesAverage Weighted Atomic MassmamazookeeprNo ratings yet
- PopcornDocument2 pagesPopcornmamazookeeprNo ratings yet
- Lab Report Format: Title Source Name Partner Date PurposeDocument2 pagesLab Report Format: Title Source Name Partner Date PurposemamazookeeprNo ratings yet
- Laboratory Exercise IV: Density LabDocument2 pagesLaboratory Exercise IV: Density LabmamazookeeprNo ratings yet
- Laboratory Exercise III: Significant FiguresDocument1 pageLaboratory Exercise III: Significant FiguresmamazookeeprNo ratings yet
- Significant Figures Jokes 1Document2 pagesSignificant Figures Jokes 1mamazookeeprNo ratings yet
- Significant Figures Jokes 2Document2 pagesSignificant Figures Jokes 2mamazookeeprNo ratings yet
Unit 6 Review
Unit 6 Review
Uploaded by
mamazookeepr0 ratings0% found this document useful (0 votes)
33 views2 pagesThis document provides a review of key concepts in chemistry including:
1) How to label the parts of a chemical reaction including reactants, products, and the arrow to show the reaction occurs.
2) Definitions of common chemistry terms like stoichiometry, catalysts, and reaction rate.
3) Factors that affect the rate of a chemical reaction such as concentration, temperature, and surface area.
4) How to analyze energy diagrams to determine if heat is absorbed or released by a reaction.
5) Practice balancing chemical equations and identifying the type of reaction.
Original Description:
equations review
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides a review of key concepts in chemistry including:
1) How to label the parts of a chemical reaction including reactants, products, and the arrow to show the reaction occurs.
2) Definitions of common chemistry terms like stoichiometry, catalysts, and reaction rate.
3) Factors that affect the rate of a chemical reaction such as concentration, temperature, and surface area.
4) How to analyze energy diagrams to determine if heat is absorbed or released by a reaction.
5) Practice balancing chemical equations and identifying the type of reaction.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
33 views2 pagesUnit 6 Review
Unit 6 Review
Uploaded by
mamazookeeprThis document provides a review of key concepts in chemistry including:
1) How to label the parts of a chemical reaction including reactants, products, and the arrow to show the reaction occurs.
2) Definitions of common chemistry terms like stoichiometry, catalysts, and reaction rate.
3) Factors that affect the rate of a chemical reaction such as concentration, temperature, and surface area.
4) How to analyze energy diagrams to determine if heat is absorbed or released by a reaction.
5) Practice balancing chemical equations and identifying the type of reaction.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 2
Unit 6 Review
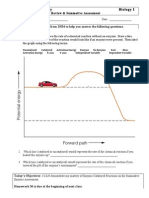
Use the diagram below to label the indicators of a chemical reaction.
C
H
E
M
I
C
A
L
Use the diagram below to label the parts of a chemical reaction.
A + B
______________
C + D
___________
________________
Definitions. Write the word that corresponds to each definition.
1.
2.
3.
4.
5.
6.
7.
8.
Shows the number of atoms in a molecule
Stirring a reaction
Speed of a reaction
Amount of substance in a given space
Using symbols to represent a reaction
Shows the number of molecules
A process that makes a new substance
A substance that speeds up a reaction
List three factors that affect reaction rate.
1. _________________________________________
2. _________________________________________
3. _________________________________________
Answer the questions about the graphs above.
1. In which graph do the products have less energy? ____________________
2. In which graph are the reactants lower in energy than the products?
__________________________
3. In which graph is heat being given off to the system? ____________________
4. In which graph do the reactants have to lose energy? ____________________
5. At what point on the graph does the reaction occur? ____________________
Balance the following equations. Then, tell the type of reaction.
Equation
Type
1. _____ NCl3 _____ N2 + _____ Cl2
6. ___________
2. _____ F2 + _____ NaI _____ NaF + _____ I2
7. ___________
3. _____ Fe + _____ O2 _____ Fe2O3
8. ___________
4. ____ HNO3 + ____ Ca(OH)2 ____ Ca(NO3)2 + ____ H2O 9. ___________
5. _____ Zn + _____ HCl _____ ZnCl2 + _____ H2
10. ___________
You might also like
- Chapter 1 - Study GuideDocument7 pagesChapter 1 - Study Guidesarahleeabc100% (1)
- Mid Year Exam Chemistry Form 4Document13 pagesMid Year Exam Chemistry Form 4Wan Shuhaimi73% (11)
- Sample Lab ReportDocument3 pagesSample Lab Reportmamazookeepr63% (8)
- Practice Exam 2 Organic Chemistry 237 UWDocument7 pagesPractice Exam 2 Organic Chemistry 237 UWNgoc Minh NgoNo ratings yet
- Activity SheetDocument3 pagesActivity Sheetjanice alquizar100% (1)
- Experimental Design POGIL 2013Document3 pagesExperimental Design POGIL 2013Steffi Basnet0% (1)
- Enzyme Graphing Worksheet: NameDocument2 pagesEnzyme Graphing Worksheet: NamePBFNo ratings yet
- Name - Date - PeriodDocument2 pagesName - Date - PeriodmamazookeeprNo ratings yet
- BIOCATALYSISDocument4 pagesBIOCATALYSISanis azizNo ratings yet
- Types of Chemical RX WSHDocument1 pageTypes of Chemical RX WSHLeonardo SierraNo ratings yet
- Eche0807 at C1Document6 pagesEche0807 at C1Hema LataNo ratings yet
- Chemical ReactionDocument4 pagesChemical ReactionABDULRAHMAN ABDRABBONo ratings yet
- 2022 Chemistry Paper I (Theory) MJ CLUSTERDocument11 pages2022 Chemistry Paper I (Theory) MJ CLUSTERishmaeljonas681No ratings yet
- Activation EnergyDocument13 pagesActivation EnergydrtanilbabuNo ratings yet
- Chemistry Mcse PiDocument10 pagesChemistry Mcse PiMoses Samalani100% (1)
- RateofReactionsQuiz 1Document1 pageRateofReactionsQuiz 1Mulki MohamedNo ratings yet
- Cellular Respiration QuestionsDocument2 pagesCellular Respiration Questionstestingacc177No ratings yet
- Microsoft Word - CH 12 Worksheet 1-3 - DocDocument8 pagesMicrosoft Word - CH 12 Worksheet 1-3 - DocMichelle NgNo ratings yet
- Chemistry Unit 7 Test: - /32 Points PossibleDocument6 pagesChemistry Unit 7 Test: - /32 Points Possibleapi-516602929No ratings yet
- Year 10 Chemistry Time: 2 HoursDocument12 pagesYear 10 Chemistry Time: 2 HoursAdrianHedleyNo ratings yet
- UntitledDocument16 pagesUntitledMichel ElizeeNo ratings yet
- Organic HgcdsynthesisDocument10 pagesOrganic HgcdsynthesisTannishk MankarNo ratings yet
- 2022 DEDZA MOCK EXAMS (CHEMIS Paper 1Document10 pages2022 DEDZA MOCK EXAMS (CHEMIS Paper 1hamzahbrave28No ratings yet
- Year 10 Chemistry Time: 2 HoursDocument9 pagesYear 10 Chemistry Time: 2 HoursAdrianHedleyNo ratings yet
- Rates of Reaction - Mini LabsDocument7 pagesRates of Reaction - Mini LabsPolly LeungNo ratings yet
- Enzyme Graphing Worksheet FormativeDocument3 pagesEnzyme Graphing Worksheet FormativeVALENTINA PEÑALOZA HERRERANo ratings yet
- PSCH 0814 KeyDocument2 pagesPSCH 0814 KeyJan Ira RenolayanNo ratings yet
- 2.8.5.c WorksheetDocument2 pages2.8.5.c WorksheetAftab AhmedNo ratings yet
- Q4 W7 8 Sci10 LawDocument8 pagesQ4 W7 8 Sci10 LawBa BengNo ratings yet
- Las Co2Document4 pagesLas Co2angel pranadaNo ratings yet
- Pre-Lab Template - 230507 - 174440Document19 pagesPre-Lab Template - 230507 - 174440WAN AZALEEYA BINTI WAN AZANI / UPMNo ratings yet
- Chapter 7 Chemical ReactionsDocument10 pagesChapter 7 Chemical Reactionsapi-30718309No ratings yet
- Description Penerangan Temperature Suhu (°c)Document4 pagesDescription Penerangan Temperature Suhu (°c)Norlailatulakma BolhassanNo ratings yet
- CollisionTheorySE Revised For MYP-GizmosDocument9 pagesCollisionTheorySE Revised For MYP-GizmosNo HumorNo ratings yet
- PAT Bio f4 P2 2015Document14 pagesPAT Bio f4 P2 2015unieezzNo ratings yet
- ds24 Enzyme Review AssessmentDocument8 pagesds24 Enzyme Review Assessmentapi-110789702No ratings yet
- 6.2 and 6.3 Worksheet Factors and ExplainationDocument2 pages6.2 and 6.3 Worksheet Factors and ExplainationhairtNo ratings yet
- A-Level Biology Protein Exam QuestionsDocument27 pagesA-Level Biology Protein Exam Questionsfreyamartin90676No ratings yet
- c6 The Rate and Extent of Chemical Change HTDocument73 pagesc6 The Rate and Extent of Chemical Change HTewfjehwjfNo ratings yet
- Ujian Setara 2 Kimia Form4Document2 pagesUjian Setara 2 Kimia Form4adikmukNo ratings yet
- Final Term Practice Work Sheet 1aDocument2 pagesFinal Term Practice Work Sheet 1aKhondokar TarakkyNo ratings yet
- Student Exploration: Collision TheoryDocument7 pagesStudent Exploration: Collision TheoryNicholas CrowellNo ratings yet
- Chemistry Time Allowed: 1 Hour Paper 2 Theory Total Marks: /45Document7 pagesChemistry Time Allowed: 1 Hour Paper 2 Theory Total Marks: /45Salman Ul MoazzamNo ratings yet
- Physical and Chemical Changes WorksheetDocument4 pagesPhysical and Chemical Changes WorksheetAndrew ChenNo ratings yet
- NewDocument1 1Document18 pagesNewDocument1 1Paweł KorczakNo ratings yet
- Activity No. 2 & 3 Structure in Organic Compounds: Use of Molecular Models Report SheetDocument2 pagesActivity No. 2 & 3 Structure in Organic Compounds: Use of Molecular Models Report Sheetlara skwngrNo ratings yet
- Enzyme Work SheetDocument6 pagesEnzyme Work SheetmdonohueHGHSNo ratings yet
- Lesson 1 - Energy and States of Matter-7apDocument2 pagesLesson 1 - Energy and States of Matter-7apunicornn2990No ratings yet
- ds36 Enzyme Review Summative AssessmentDocument4 pagesds36 Enzyme Review Summative Assessmentapi-110789702No ratings yet
- Worksheet 8.3 (Ionic Equation Step by Step)Document2 pagesWorksheet 8.3 (Ionic Equation Step by Step)ChantoniNo ratings yet
- IBO 2008 Mumbai Theory Examination 2Document80 pagesIBO 2008 Mumbai Theory Examination 2Ari GaoNo ratings yet
- ch21 1readingassignmentDocument2 pagesch21 1readingassignmentapi-324905565No ratings yet
- The Periodic Table WorksheetDocument1 pageThe Periodic Table WorksheetZ KNo ratings yet
- Organic 1Document6 pagesOrganic 1Jaspar GlagovsNo ratings yet
- Workshop - Fuels-Fractional Distillation-CrackingDocument4 pagesWorkshop - Fuels-Fractional Distillation-Crackingmeganekokun kawaiiNo ratings yet
- Ict6-Worksheet-No.6 With Key AnswerDocument3 pagesIct6-Worksheet-No.6 With Key Answerjhe azulNo ratings yet
- Covalent Substances TestDocument7 pagesCovalent Substances TestJaspar GlagovsNo ratings yet
- Use Your Notes From The Atomic Structure Program To Answer The Following QuestionsDocument1 pageUse Your Notes From The Atomic Structure Program To Answer The Following QuestionsAngieOrtegaNo ratings yet
- AtomstructurewkstDocument1 pageAtomstructurewkstapi-270967967No ratings yet
- Grade 9 Technology Final ExamDocument8 pagesGrade 9 Technology Final ExamLaila KaderNo ratings yet
- Hydrate Lab: Never Place A Hot Crucible On A Balance! When Cool, Determine The Mass of The Crucible andDocument1 pageHydrate Lab: Never Place A Hot Crucible On A Balance! When Cool, Determine The Mass of The Crucible andmamazookeeprNo ratings yet
- Build A Trend LabDocument2 pagesBuild A Trend LabmamazookeeprNo ratings yet
- Chemical Formula QuizDocument3 pagesChemical Formula QuizmamazookeeprNo ratings yet
- Unit 4 Test 2009Document2 pagesUnit 4 Test 2009mamazookeeprNo ratings yet
- Subatomic Particle WorksheetDocument1 pageSubatomic Particle WorksheetmamazookeeprNo ratings yet
- Average Weighted Atomic MassDocument2 pagesAverage Weighted Atomic MassmamazookeeprNo ratings yet
- PopcornDocument2 pagesPopcornmamazookeeprNo ratings yet
- Lab Report Format: Title Source Name Partner Date PurposeDocument2 pagesLab Report Format: Title Source Name Partner Date PurposemamazookeeprNo ratings yet
- Laboratory Exercise IV: Density LabDocument2 pagesLaboratory Exercise IV: Density LabmamazookeeprNo ratings yet
- Laboratory Exercise III: Significant FiguresDocument1 pageLaboratory Exercise III: Significant FiguresmamazookeeprNo ratings yet
- Significant Figures Jokes 1Document2 pagesSignificant Figures Jokes 1mamazookeeprNo ratings yet
- Significant Figures Jokes 2Document2 pagesSignificant Figures Jokes 2mamazookeeprNo ratings yet