Professional Documents
Culture Documents
ABG Interpretation: Respiratory System
ABG Interpretation: Respiratory System
Uploaded by
Arsalan NadeemOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ABG Interpretation: Respiratory System
ABG Interpretation: Respiratory System
Uploaded by
Arsalan NadeemCopyright:
Available Formats
ABG Interpretation
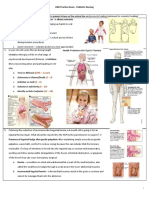
Look at pH, CO2, and HCO3. pH determines whether it is alkalosis or acidosis. Normal is 7.35-7.45, low < 7.35 is acidotic, values < 7.2 is critically low and > 7.6 is critically high, life threatening. PaCO2 is carbon dioxide, what pts exhale Normal is 35-45. It is the acid in the acid/base balance. HCO3 is Bicarbonate, what pts' kidneys put out to act as the buffer or base. Normal is 22-26. It is the base in the acid/base balance.
Respiratory System
PaCO2 < 35 shows alkalosis. This will raise the pH. It is usually caused by increasing rate or volume of breathing. - Tx by decreasing rate or volume. PaCO2 > 45 shows acidosis. This will lower the pH. It is usually caused by decreasing rate or volume of breathing. Restrictive Lung Disease will produce this COPD, asthma, bronchitis, etc. Tx this by increasing rate or volume Remember pH is the determining factor. If pH is normal, the acid/base balance may be compensating for changes in CO2 or HCO3.
Metabolic System
HCO3 < 22 shows acidosis. This will lower the pH. This might indicate decrease in renal function, post-op heart surgery, large blood transfusions. - Tx is with NaHCO3. HCO3 > 26 shows alkalosis. This will raise the pH. Causes include diarrhea or large gastric loss. - Tx is to tx underlying problem. Remember pH is the determining factor. Look at the pH, first.
Determining the Problem
Look first at pH. It is acidosis < 7.35 or alkalosis > 7.45 or is it normal. Then look at the PaCO2 and the HCO3 to see which one is causing the pH to change or both. If pH is acidosis and PaCO2 is high, it is respiratory acidosis. If pH is acidosis and HCO3 is low, it is metabolic acidosis.
Determining the Problem
If the pH is high, alkalosis, then look at CO2, if it is low, then it is respiratory alkalosis. If the pH is high, alkalosis, then look at HCO3, if it is high, then it is metabolic alkalosis. If pH is normal but the CO2 and the HCO3 are abnormal, we know then the body is compensating and we see which one is causing the pH to change closer to its side (acid or base).
Studies
Case 1 Normal Values Case 2 PH 7.28 7.35-7.45 PH 7.36 PaO2 85 80 -100 PaO2 139 PaCO2 65 35-45 PaCO2 51 HCO3 25 22-26 HCO3 28 BE is base excess is acidotic + is alkalotic Case 3 PH 7.52 PaO2 75 PaCO2 35 HCO3 37 7.35-7.45 80 100 35 45 22 26
You might also like
- NCLEX Cram SheetDocument6 pagesNCLEX Cram Sheetaishwariyapokharel55No ratings yet
- Fluid and Electrolytes Made Insanely Easy PDFDocument20 pagesFluid and Electrolytes Made Insanely Easy PDFCherry Ann Garcia Durante100% (7)
- Business Research Methods Zikmund 9th Edition TestbankDocument2 pagesBusiness Research Methods Zikmund 9th Edition TestbankArsalan Nadeem50% (2)
- Live NCLEX Review Lecture Slides-2Document510 pagesLive NCLEX Review Lecture Slides-2Ronny Andres Carrasco100% (10)
- NCLEX RN Review For Reduction of Risk PotentialDocument7 pagesNCLEX RN Review For Reduction of Risk Potentialpaulinatia100% (1)
- Nclex MedsssDocument6 pagesNclex MedsssSheryl_13No ratings yet
- LPN DELEGATION and PRIORITIZING AND MOREDocument38 pagesLPN DELEGATION and PRIORITIZING AND MOREDebra PowellNo ratings yet
- Exit Hesi Study Plan #1 10 5 11Document1 pageExit Hesi Study Plan #1 10 5 11mmgoodall22100% (1)
- Comprehensive Nclex Notes Easy To Read PDFDocument97 pagesComprehensive Nclex Notes Easy To Read PDFKenia GeorgesNo ratings yet
- By Sandhya KumarDocument20 pagesBy Sandhya KumarKhalid ShafiqNo ratings yet
- RN Intense Remedial Packet AnswersDocument53 pagesRN Intense Remedial Packet AnswersAli Resendiz60% (5)
- MedicalSurgical Nursing ReviewDocument86 pagesMedicalSurgical Nursing ReviewPopa D. SilviuNo ratings yet
- BURNSDocument4 pagesBURNSRizMarie100% (2)
- Hurst 11 - RenalDocument6 pagesHurst 11 - RenalPascal St Peter NwaorguNo ratings yet
- NCLEX - Acid Base BalanceDocument1 pageNCLEX - Acid Base BalanceNathalee WalkerNo ratings yet
- Nclex Disease: 4. Oral ContraceptivesDocument7 pagesNclex Disease: 4. Oral ContraceptivesMeijia SongNo ratings yet
- Nclex 3500Document4 pagesNclex 3500chitorNo ratings yet
- A-T-I Endocrine NotesDocument3 pagesA-T-I Endocrine NotesKelseyAnnBarnesNo ratings yet
- ATI Comprehensive Predictor: Study Online atDocument9 pagesATI Comprehensive Predictor: Study Online atVanessaMUellerNo ratings yet
- Peds Unit 2 Study GuideDocument25 pagesPeds Unit 2 Study GuideKyptasticNo ratings yet
- Nclex NotesDocument18 pagesNclex Notesmaane1005No ratings yet
- Musculoskeletal Note1Document34 pagesMusculoskeletal Note1FreeNursingNotes100% (4)
- Concept Map Et Al 11-04-15Document7 pagesConcept Map Et Al 11-04-15api-353656227No ratings yet
- NCLEX Pregnancy NotesDocument3 pagesNCLEX Pregnancy NotesrustiejadeNo ratings yet
- Select All That ApplyDocument10 pagesSelect All That ApplyJohnasse Sebastian NavalNo ratings yet
- NCLEXstudyguidevmDocument31 pagesNCLEXstudyguidevmAnonymous vqG0paWG100% (1)
- NCLEX Medical Terminology ReviewDocument2 pagesNCLEX Medical Terminology ReviewFilipino Nurses CentralNo ratings yet
- ECG Interpretation: Learning The Basics: Presented byDocument65 pagesECG Interpretation: Learning The Basics: Presented bySherry DoddsNo ratings yet
- ABG ROME FlowchartDocument1 pageABG ROME FlowchartRoshani PatelNo ratings yet
- Fluids and Electrolytes: Irene L. Gardiner, MDDocument48 pagesFluids and Electrolytes: Irene L. Gardiner, MDGabriel Carlo FranciscoNo ratings yet
- Med Surg2 PDFDocument19 pagesMed Surg2 PDFHasan A AsFourNo ratings yet
- ABG Tic Tac Toe Part 2Document2 pagesABG Tic Tac Toe Part 2C Caballero LobregasNo ratings yet
- 4x6 Module 6 Drug CardsDocument10 pages4x6 Module 6 Drug CardsQueennitaNo ratings yet
- Nclex BulletsDocument34 pagesNclex Bulletssaroberts2202100% (1)
- Peds Ati - VS + Lab ValuesDocument1 pagePeds Ati - VS + Lab Valuesluke jackson100% (1)
- IV PDFDocument63 pagesIV PDFelbagouryNo ratings yet
- Hurst - Content Review - Cardio (Edit)Document8 pagesHurst - Content Review - Cardio (Edit)Elaine NorbergNo ratings yet
- The Client Should Be Instructed As Follows: Intravenous Pyelogram (IVP) Is An X-Ray of The Urinary SystemDocument25 pagesThe Client Should Be Instructed As Follows: Intravenous Pyelogram (IVP) Is An X-Ray of The Urinary SystemElizabeth SharmaNo ratings yet
- Infection Control For NclexDocument9 pagesInfection Control For NclexMadora SamuelNo ratings yet
- Open Pediatric ArcherDocument84 pagesOpen Pediatric Archerbaharada1979No ratings yet
- ATI NotesDocument16 pagesATI NotesMei Sarte100% (1)
- My Hesi Study GuideDocument21 pagesMy Hesi Study GuidejujusmallsNo ratings yet
- Week 3 Nclex ReviewDocument71 pagesWeek 3 Nclex Reviewmelissamichellle50% (2)
- Cardiovascular System Acronyms and MnemonicsDocument18 pagesCardiovascular System Acronyms and Mnemonicsdecsag06No ratings yet
- Lab ValuesDocument14 pagesLab Valuessylwiasajna100% (2)
- Immunity 1Document6 pagesImmunity 1Tori RolandNo ratings yet
- Final Peds Concept MapDocument9 pagesFinal Peds Concept Mapapi-495456666No ratings yet
- Medical-Surgical Nursing: Perioperative OverviewDocument24 pagesMedical-Surgical Nursing: Perioperative OverviewSheena Ann L. LLarenasNo ratings yet
- My Cheat SheetDocument3 pagesMy Cheat SheetTenzin KyizomNo ratings yet
- Paed Parenteral Fluid ThyDocument1 pagePaed Parenteral Fluid ThyZulia Ahmad BurhaniNo ratings yet
- HESI Exam PracticeDocument33 pagesHESI Exam PracticeJean AustenNo ratings yet
- Kaplan Focus ReviewDocument9 pagesKaplan Focus ReviewSaidel ElizondoNo ratings yet
- Maternity Ati RemediationDocument2 pagesMaternity Ati Remediationcse100% (1)
- NCLEX OB Peds 2 of 3 - 947 TermsDocument243 pagesNCLEX OB Peds 2 of 3 - 947 TermsCharaNo ratings yet
- NURSING CARE OF ADULTS I: Passbooks Study GuideFrom EverandNURSING CARE OF ADULTS I: Passbooks Study GuideNo ratings yet
- ABG AnalysisDocument7 pagesABG Analysissneha100% (1)
- Simple Method of Acid Base Balance InterpretationDocument11 pagesSimple Method of Acid Base Balance InterpretationChin ChanNo ratings yet
- Arterial Blood Gas (ABG) AnalysisDocument18 pagesArterial Blood Gas (ABG) AnalysisAnandhu GNo ratings yet
- Simple Method of ABGs InterpretationDocument9 pagesSimple Method of ABGs Interpretationjhorn_appleNo ratings yet
- Y5 RN - Abgs 27oct2010Document5 pagesY5 RN - Abgs 27oct2010Canh VanNo ratings yet
- Robert Louis Balfour StevensonDocument1 pageRobert Louis Balfour StevensonArsalan NadeemNo ratings yet
- Literature ReviewDocument7 pagesLiterature ReviewArsalan NadeemNo ratings yet
- Literature ReviewDocument7 pagesLiterature ReviewArsalan NadeemNo ratings yet
- CH 06Document5 pagesCH 06Arsalan NadeemNo ratings yet
- Institute of Business Management: Seminar in Economic Polices Thesis Paper OnDocument19 pagesInstitute of Business Management: Seminar in Economic Polices Thesis Paper OnArsalan NadeemNo ratings yet
- Acknowledgement: We Would Like To Thank Sir Fakhir MusharrafDocument6 pagesAcknowledgement: We Would Like To Thank Sir Fakhir MusharrafArsalan NadeemNo ratings yet
- Abg Interpretation: Adam Cooper, RN, MSN Nursing EducationDocument40 pagesAbg Interpretation: Adam Cooper, RN, MSN Nursing EducationArsalan NadeemNo ratings yet
- Total Annual Budget: EstimatedDocument5 pagesTotal Annual Budget: EstimatedArsalan NadeemNo ratings yet
- RecommendationDocument11 pagesRecommendationArsalan NadeemNo ratings yet
- Dengue Fever: By:Mehreen (09-100)Document20 pagesDengue Fever: By:Mehreen (09-100)Arsalan NadeemNo ratings yet
- Case PresentationDocument31 pagesCase PresentationArsalan NadeemNo ratings yet
- Approach To HematuriaDocument15 pagesApproach To HematuriaArsalan NadeemNo ratings yet
- 22 - Proteinuria and HematuriaDocument73 pages22 - Proteinuria and HematuriaArsalan NadeemNo ratings yet
- Presidential Immunity 1Document4 pagesPresidential Immunity 1Arsalan NadeemNo ratings yet
- Picasso Company Profit and Loss Account AS AT DEC 31, 2013Document3 pagesPicasso Company Profit and Loss Account AS AT DEC 31, 2013Arsalan NadeemNo ratings yet