Professional Documents
Culture Documents
C1B Singly Bonded Functional Groups. Tutorial Questions Spring 2014
C1B Singly Bonded Functional Groups. Tutorial Questions Spring 2014
Uploaded by
Jan Hroch KošataCopyright:
Available Formats
You might also like
- IGCSE Coordinated Science Keyword Definitions Master Copy 20-3-12Document13 pagesIGCSE Coordinated Science Keyword Definitions Master Copy 20-3-12Dhingra shelly100% (1)
- BIOKMOR N01 3rd ExamDocument8 pagesBIOKMOR N01 3rd ExamMacy MarianNo ratings yet
- 11 AllDocument28 pages11 AllEdson EmidioNo ratings yet
- Atomic BasicsDocument4 pagesAtomic Basicsapi-169639475No ratings yet
- Car Wash Business Plan by SlidesgoDocument57 pagesCar Wash Business Plan by SlidesgoAngela BuilesNo ratings yet
- Homework 1 2302271 Organic Chemistry IDocument2 pagesHomework 1 2302271 Organic Chemistry IVee Worabhorn0% (1)
- SCGS F.7 AL Chemistry Assignment 2 - HALOALKANESDocument1 pageSCGS F.7 AL Chemistry Assignment 2 - HALOALKANESsachinkurhekarNo ratings yet
- WORK BOOK - Exercise in ChemistryDocument28 pagesWORK BOOK - Exercise in ChemistryTikeshwar SharmaNo ratings yet
- HC Docx1Document13 pagesHC Docx1ayushsekhariNo ratings yet
- Practice Problems On Addition Reactions To Alkenes With AnswersDocument4 pagesPractice Problems On Addition Reactions To Alkenes With AnswersSangetha ChelladoraiNo ratings yet
- ch9 AlkynesDocument7 pagesch9 AlkynesApichat JunsodNo ratings yet
- Chapter 6 Properties of HaloalkaneDocument5 pagesChapter 6 Properties of HaloalkaneRen Liew Jia QingNo ratings yet
- CHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsDocument15 pagesCHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsSara Yuen100% (1)
- Tugas - 6 Senyawa AlkunaDocument3 pagesTugas - 6 Senyawa AlkunaBaiq ArinNo ratings yet
- Unit-12 Aldehyde Ketone Carboxylic AcidDocument5 pagesUnit-12 Aldehyde Ketone Carboxylic AcidVIDHI CHORDIANo ratings yet
- Nitrogen Containing Compuonds-03 - Assignments (New)Document20 pagesNitrogen Containing Compuonds-03 - Assignments (New)Raju SinghNo ratings yet
- Chapter 7Document30 pagesChapter 7Apichat Junsod100% (4)
- QB - Aldehydes, Ketones and Carboxylic AcidsDocument5 pagesQB - Aldehydes, Ketones and Carboxylic AcidsAkshith ReddyNo ratings yet
- I IIT (IRP) Chemistry Worksheet - 16Document9 pagesI IIT (IRP) Chemistry Worksheet - 16Ashwin KumarNo ratings yet
- Massachusetts Institute of Technology 5.12, Spring 2005: Problem Set #4Document9 pagesMassachusetts Institute of Technology 5.12, Spring 2005: Problem Set #4Minh TieuNo ratings yet
- Aldehyde, Ketone and Carboxylic AcidDocument10 pagesAldehyde, Ketone and Carboxylic Acidgoodgirlz946No ratings yet
- Acfrogbyyb W54zpzfswkn8k3vq0clq6et8mk Ne Px62hvrlk5chrlql9xx83xtq2sr0dqcuhrswcoglr Ueky068cras4ph7jxkmy 143kq0wnhekbynbh 4 Eq1p0kvslajoriecir6ikqqswDocument8 pagesAcfrogbyyb W54zpzfswkn8k3vq0clq6et8mk Ne Px62hvrlk5chrlql9xx83xtq2sr0dqcuhrswcoglr Ueky068cras4ph7jxkmy 143kq0wnhekbynbh 4 Eq1p0kvslajoriecir6ikqqswThanh Hằng NgôNo ratings yet
- Chapter 1 ReviewDocument2 pagesChapter 1 ReviewGmat PrepNo ratings yet
- CU-2020 B.Sc. (Honours) Chemistry Semester-III Paper-CC-7 QPDocument4 pagesCU-2020 B.Sc. (Honours) Chemistry Semester-III Paper-CC-7 QPbuntyckbtNo ratings yet
- UAB Wang Recitation #1Document4 pagesUAB Wang Recitation #1OmarBilbeisiNo ratings yet
- Moc 5001 2016Document9 pagesMoc 5001 2016Anne Nirmani RodrigoNo ratings yet
- CHM096-Tutorial 2 (Alcohols Etc.)Document5 pagesCHM096-Tutorial 2 (Alcohols Etc.)Anonymous RD1CrAINo ratings yet
- Chemistry 101 Practice EXAM ANSWERSDocument25 pagesChemistry 101 Practice EXAM ANSWERSSakinah AzmiNo ratings yet
- Tutorial 6 (Alcohols, Aldehydes, Haloalkanes, Carboxylic Acids)Document5 pagesTutorial 6 (Alcohols, Aldehydes, Haloalkanes, Carboxylic Acids)dasani93No ratings yet
- 12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS QDocument20 pages12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS Q123No ratings yet
- Su 99 Org 1 FinalDocument24 pagesSu 99 Org 1 FinalPhương Nail TócNo ratings yet
- Chem Book 2 TestDocument3 pagesChem Book 2 TestHishq DhimanNo ratings yet
- CL CL: Hex-1-En-4-Yne or 1-Hexen-4-YneDocument4 pagesCL CL: Hex-1-En-4-Yne or 1-Hexen-4-YneSamuel Espinoza GarciaNo ratings yet
- Final Exam For Organic II 200pts (Weighted As 300) : ROH ROR RCNDocument23 pagesFinal Exam For Organic II 200pts (Weighted As 300) : ROH ROR RCNUmmi Khairani UrfaNo ratings yet
- ALDEHYDES, KETONES, ACIDS-01-170419: Neet-Crash-2017 Chemistry TestDocument6 pagesALDEHYDES, KETONES, ACIDS-01-170419: Neet-Crash-2017 Chemistry TestPoorvaBakshiNo ratings yet
- Organic Chemistry Additional Problems Final Exam Part2Document6 pagesOrganic Chemistry Additional Problems Final Exam Part2John SmithNo ratings yet
- Aldehydes & KetonesDocument23 pagesAldehydes & KetonesManthan JhaNo ratings yet
- CHM133 PRELIM EXAM Part2Document7 pagesCHM133 PRELIM EXAM Part2Rohaisa FaisalNo ratings yet
- SP 2006 Final Examination Organic II 200pts (Weighted As 300)Document22 pagesSP 2006 Final Examination Organic II 200pts (Weighted As 300)Ummi Khairani UrfaNo ratings yet
- Acids and Derivatives TutorialDocument18 pagesAcids and Derivatives TutorialChen ZhihaoNo ratings yet
- Chemistry Unit 2Document12 pagesChemistry Unit 2kelon scottNo ratings yet
- Additional Problems Final Exam Part 2 AnswersDocument10 pagesAdditional Problems Final Exam Part 2 AnswersJohn SmithNo ratings yet
- Organic ChemistryDocument45 pagesOrganic ChemistryAnubhav Sinha0% (1)
- SP 2004 Final Organic II 200pts (Weighted As 300) : RNH C N H O RN +Document25 pagesSP 2004 Final Organic II 200pts (Weighted As 300) : RNH C N H O RN +Ummi Khairani UrfaNo ratings yet
- Alcohols, Phenols and EthersDocument18 pagesAlcohols, Phenols and Etherssaksham05060% (1)
- HW2 2013Document3 pagesHW2 2013kitty2911No ratings yet
- Assignment 1 - Aldehyde and Ketone Mac-Jul 2013Document2 pagesAssignment 1 - Aldehyde and Ketone Mac-Jul 2013anessismanisNo ratings yet
- Alkyl Halides, Alcohols, Ethers and Epoxides: 1. What Is The IUPAC Name For CHDocument17 pagesAlkyl Halides, Alcohols, Ethers and Epoxides: 1. What Is The IUPAC Name For CHEllaŠtrbacNo ratings yet
- 09-Final With SolutionsDocument27 pages09-Final With SolutionsDanielle Wood100% (2)
- Chemistry AminesDocument8 pagesChemistry AminesVanshika LudhaniNo ratings yet
- Aldehydes, Ketones and Carboxylic AcidsDocument7 pagesAldehydes, Ketones and Carboxylic Acidskavitha2511977No ratings yet
- Diwali Assignment 12thDocument19 pagesDiwali Assignment 12thNishantPlayz YtNo ratings yet
- Organic SimplificationDocument13 pagesOrganic SimplificationKaushal Silva RanpatabendigeNo ratings yet
- M.SC 1st Sem Unit-2 2015Document6 pagesM.SC 1st Sem Unit-2 2015bgroyNo ratings yet
- SP 2007 Final Examination Organic II 200pts (Weighted As 300)Document21 pagesSP 2007 Final Examination Organic II 200pts (Weighted As 300)Ummi Khairani UrfaNo ratings yet
- Tutorial 3Document8 pagesTutorial 3Ahmad WahideeNo ratings yet
- Chemistry s5 Theory and Pract.Document29 pagesChemistry s5 Theory and Pract.ngabonzizayusuf9No ratings yet
- Graphene Oxide: Fundamentals and ApplicationsFrom EverandGraphene Oxide: Fundamentals and ApplicationsAyrat M. DimievNo ratings yet
- Arene Chemistry: Reaction Mechanisms and Methods for Aromatic CompoundsFrom EverandArene Chemistry: Reaction Mechanisms and Methods for Aromatic CompoundsNo ratings yet
- Chapter - 5dnonlinear Acoustic Propagation PDFDocument7 pagesChapter - 5dnonlinear Acoustic Propagation PDFAlinaBogoiNo ratings yet
- GE2023 Fundamentals of Nanoscience L T P C3 0 0 3 Unit I 9Document36 pagesGE2023 Fundamentals of Nanoscience L T P C3 0 0 3 Unit I 9yokeshNo ratings yet
- FYP List 2020 21RDocument3 pagesFYP List 2020 21RSaif UllahNo ratings yet
- Kinetics of A Particle Impulse and MomentumDocument17 pagesKinetics of A Particle Impulse and MomentumTejada, Brent LesterNo ratings yet
- 2 Methods of PolymerizationDocument13 pages2 Methods of Polymerizationbt21102047 Vishwajeet YadavNo ratings yet
- Narayana: Common Practice Test-04Document8 pagesNarayana: Common Practice Test-04narayana dwarkaNo ratings yet
- Baysolvex D2ehpa D2ehp eDocument4 pagesBaysolvex D2ehpa D2ehp evivianaramosmerinoNo ratings yet
- Hach Sample CellsDocument8 pagesHach Sample CellsKania LouisNo ratings yet
- Candidates Are Required To Give Their Answers in Their Own Words As Far As Practicable. The Figures in The Margin Indicate Full MarksDocument4 pagesCandidates Are Required To Give Their Answers in Their Own Words As Far As Practicable. The Figures in The Margin Indicate Full MarksAdhikari SushilNo ratings yet
- Electrochemical Energy-Part1Document59 pagesElectrochemical Energy-Part1GAYOSO, CHARLES F.No ratings yet
- 8-1 Worksheet PressureDocument2 pages8-1 Worksheet PressureGuillermo José Verbel MartínezNo ratings yet
- Workshet For Pre Board 1 XII 17-18Document4 pagesWorkshet For Pre Board 1 XII 17-18Sunita NinganurNo ratings yet
- RF Sputtering Manual 2010Document23 pagesRF Sputtering Manual 2010sinytells0% (1)
- Angels International CollegeDocument10 pagesAngels International CollegeTahira ZahidNo ratings yet
- Sulfur Slide ShowDocument14 pagesSulfur Slide ShowAbdulRehmanNo ratings yet
- Fiberline Screenroom Improvements and The Benefits in Pulp QualityDocument5 pagesFiberline Screenroom Improvements and The Benefits in Pulp QualityBastian Richard SitohangNo ratings yet
- Cahn-Ingold-Prelog Priority RulesDocument5 pagesCahn-Ingold-Prelog Priority RulesBer GuzNo ratings yet
- Multiphase Systems Containing A Component: Single CondensableDocument35 pagesMultiphase Systems Containing A Component: Single Condensableibrahim3318No ratings yet
- Bruce E. Depalma: N-Machine: Extraction of Electrical Energy Directly From Space: The N-MachineDocument7 pagesBruce E. Depalma: N-Machine: Extraction of Electrical Energy Directly From Space: The N-MachineHaris DizdarevicNo ratings yet
- Worksheet SAtoVratioworksheetwithkeyDocument2 pagesWorksheet SAtoVratioworksheetwithkeynim35No ratings yet
- Transient Heat Conduction: Course ContentsDocument13 pagesTransient Heat Conduction: Course ContentsJainil GajjarNo ratings yet
- F 2282 - 03 - RjiyodiDocument15 pagesF 2282 - 03 - RjiyodikrutikNo ratings yet
- Learning Unit 1 (Chap. 11)Document15 pagesLearning Unit 1 (Chap. 11)sbusisoNo ratings yet
- Electrical Circuits 1 1.1 Atomic Theory of MatterDocument12 pagesElectrical Circuits 1 1.1 Atomic Theory of MatterJohn Michael CabasaNo ratings yet
- 2021 JMRT EC RefinementDocument13 pages2021 JMRT EC Refinement김문조No ratings yet
- Energy Refractories Ammonia Methanol en 1004 TdsDocument2 pagesEnergy Refractories Ammonia Methanol en 1004 Tdsshuyang zhangNo ratings yet
- CH 31Document20 pagesCH 31Muzamil Shah100% (2)
C1B Singly Bonded Functional Groups. Tutorial Questions Spring 2014
C1B Singly Bonded Functional Groups. Tutorial Questions Spring 2014
Uploaded by
Jan Hroch KošataOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
C1B Singly Bonded Functional Groups. Tutorial Questions Spring 2014
C1B Singly Bonded Functional Groups. Tutorial Questions Spring 2014
Uploaded by
Jan Hroch KošataCopyright:
Available Formats
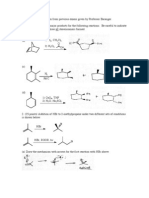
C1B Singly bonded functional groups.
Tutorial Questions
Spring 2014
1. Suggest synthetic routes to the following compounds using benzyl bromide (PhCH2Br) and any other necessary reagent as the starting materials. (a) PhCH2I (c) PhCH2NH2 (2 steps) (e) PhCH2CH2NH2 (2 steps) (g) PhCH2CCH (b) PhCH2CN (d) PhCH2SH (f) PhCH2OCH3 (h) CH3CO2CH2Ph
2.
Give the products of the following SN2 reactions, and indicate which will be optically active. Label the products R or S where appropriate.
3.
Complete the following SN1 reactions showing the intermediate carbocation in each case.
4.
For each of the following alkyl halides complete the equation showing the possible nucleophilic substitution and elimination products.
5.
How is epoxycyclohexane (A) prepared from cyclohexene?
Show, with mechanisms, the outcome of the reaction of A with: (a) (d) H3O+ NaCH / H2O (b) (e) MeOH / H+ MeONa / MeOH (c) NH3
You might also like
- IGCSE Coordinated Science Keyword Definitions Master Copy 20-3-12Document13 pagesIGCSE Coordinated Science Keyword Definitions Master Copy 20-3-12Dhingra shelly100% (1)
- BIOKMOR N01 3rd ExamDocument8 pagesBIOKMOR N01 3rd ExamMacy MarianNo ratings yet
- 11 AllDocument28 pages11 AllEdson EmidioNo ratings yet
- Atomic BasicsDocument4 pagesAtomic Basicsapi-169639475No ratings yet
- Car Wash Business Plan by SlidesgoDocument57 pagesCar Wash Business Plan by SlidesgoAngela BuilesNo ratings yet
- Homework 1 2302271 Organic Chemistry IDocument2 pagesHomework 1 2302271 Organic Chemistry IVee Worabhorn0% (1)
- SCGS F.7 AL Chemistry Assignment 2 - HALOALKANESDocument1 pageSCGS F.7 AL Chemistry Assignment 2 - HALOALKANESsachinkurhekarNo ratings yet
- WORK BOOK - Exercise in ChemistryDocument28 pagesWORK BOOK - Exercise in ChemistryTikeshwar SharmaNo ratings yet
- HC Docx1Document13 pagesHC Docx1ayushsekhariNo ratings yet
- Practice Problems On Addition Reactions To Alkenes With AnswersDocument4 pagesPractice Problems On Addition Reactions To Alkenes With AnswersSangetha ChelladoraiNo ratings yet
- ch9 AlkynesDocument7 pagesch9 AlkynesApichat JunsodNo ratings yet
- Chapter 6 Properties of HaloalkaneDocument5 pagesChapter 6 Properties of HaloalkaneRen Liew Jia QingNo ratings yet
- CHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsDocument15 pagesCHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsSara Yuen100% (1)
- Tugas - 6 Senyawa AlkunaDocument3 pagesTugas - 6 Senyawa AlkunaBaiq ArinNo ratings yet
- Unit-12 Aldehyde Ketone Carboxylic AcidDocument5 pagesUnit-12 Aldehyde Ketone Carboxylic AcidVIDHI CHORDIANo ratings yet
- Nitrogen Containing Compuonds-03 - Assignments (New)Document20 pagesNitrogen Containing Compuonds-03 - Assignments (New)Raju SinghNo ratings yet
- Chapter 7Document30 pagesChapter 7Apichat Junsod100% (4)
- QB - Aldehydes, Ketones and Carboxylic AcidsDocument5 pagesQB - Aldehydes, Ketones and Carboxylic AcidsAkshith ReddyNo ratings yet
- I IIT (IRP) Chemistry Worksheet - 16Document9 pagesI IIT (IRP) Chemistry Worksheet - 16Ashwin KumarNo ratings yet
- Massachusetts Institute of Technology 5.12, Spring 2005: Problem Set #4Document9 pagesMassachusetts Institute of Technology 5.12, Spring 2005: Problem Set #4Minh TieuNo ratings yet
- Aldehyde, Ketone and Carboxylic AcidDocument10 pagesAldehyde, Ketone and Carboxylic Acidgoodgirlz946No ratings yet
- Acfrogbyyb W54zpzfswkn8k3vq0clq6et8mk Ne Px62hvrlk5chrlql9xx83xtq2sr0dqcuhrswcoglr Ueky068cras4ph7jxkmy 143kq0wnhekbynbh 4 Eq1p0kvslajoriecir6ikqqswDocument8 pagesAcfrogbyyb W54zpzfswkn8k3vq0clq6et8mk Ne Px62hvrlk5chrlql9xx83xtq2sr0dqcuhrswcoglr Ueky068cras4ph7jxkmy 143kq0wnhekbynbh 4 Eq1p0kvslajoriecir6ikqqswThanh Hằng NgôNo ratings yet
- Chapter 1 ReviewDocument2 pagesChapter 1 ReviewGmat PrepNo ratings yet
- CU-2020 B.Sc. (Honours) Chemistry Semester-III Paper-CC-7 QPDocument4 pagesCU-2020 B.Sc. (Honours) Chemistry Semester-III Paper-CC-7 QPbuntyckbtNo ratings yet
- UAB Wang Recitation #1Document4 pagesUAB Wang Recitation #1OmarBilbeisiNo ratings yet
- Moc 5001 2016Document9 pagesMoc 5001 2016Anne Nirmani RodrigoNo ratings yet
- CHM096-Tutorial 2 (Alcohols Etc.)Document5 pagesCHM096-Tutorial 2 (Alcohols Etc.)Anonymous RD1CrAINo ratings yet
- Chemistry 101 Practice EXAM ANSWERSDocument25 pagesChemistry 101 Practice EXAM ANSWERSSakinah AzmiNo ratings yet
- Tutorial 6 (Alcohols, Aldehydes, Haloalkanes, Carboxylic Acids)Document5 pagesTutorial 6 (Alcohols, Aldehydes, Haloalkanes, Carboxylic Acids)dasani93No ratings yet
- 12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS QDocument20 pages12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS Q123No ratings yet
- Su 99 Org 1 FinalDocument24 pagesSu 99 Org 1 FinalPhương Nail TócNo ratings yet
- Chem Book 2 TestDocument3 pagesChem Book 2 TestHishq DhimanNo ratings yet
- CL CL: Hex-1-En-4-Yne or 1-Hexen-4-YneDocument4 pagesCL CL: Hex-1-En-4-Yne or 1-Hexen-4-YneSamuel Espinoza GarciaNo ratings yet
- Final Exam For Organic II 200pts (Weighted As 300) : ROH ROR RCNDocument23 pagesFinal Exam For Organic II 200pts (Weighted As 300) : ROH ROR RCNUmmi Khairani UrfaNo ratings yet
- ALDEHYDES, KETONES, ACIDS-01-170419: Neet-Crash-2017 Chemistry TestDocument6 pagesALDEHYDES, KETONES, ACIDS-01-170419: Neet-Crash-2017 Chemistry TestPoorvaBakshiNo ratings yet
- Organic Chemistry Additional Problems Final Exam Part2Document6 pagesOrganic Chemistry Additional Problems Final Exam Part2John SmithNo ratings yet
- Aldehydes & KetonesDocument23 pagesAldehydes & KetonesManthan JhaNo ratings yet
- CHM133 PRELIM EXAM Part2Document7 pagesCHM133 PRELIM EXAM Part2Rohaisa FaisalNo ratings yet
- SP 2006 Final Examination Organic II 200pts (Weighted As 300)Document22 pagesSP 2006 Final Examination Organic II 200pts (Weighted As 300)Ummi Khairani UrfaNo ratings yet
- Acids and Derivatives TutorialDocument18 pagesAcids and Derivatives TutorialChen ZhihaoNo ratings yet
- Chemistry Unit 2Document12 pagesChemistry Unit 2kelon scottNo ratings yet
- Additional Problems Final Exam Part 2 AnswersDocument10 pagesAdditional Problems Final Exam Part 2 AnswersJohn SmithNo ratings yet
- Organic ChemistryDocument45 pagesOrganic ChemistryAnubhav Sinha0% (1)
- SP 2004 Final Organic II 200pts (Weighted As 300) : RNH C N H O RN +Document25 pagesSP 2004 Final Organic II 200pts (Weighted As 300) : RNH C N H O RN +Ummi Khairani UrfaNo ratings yet
- Alcohols, Phenols and EthersDocument18 pagesAlcohols, Phenols and Etherssaksham05060% (1)
- HW2 2013Document3 pagesHW2 2013kitty2911No ratings yet
- Assignment 1 - Aldehyde and Ketone Mac-Jul 2013Document2 pagesAssignment 1 - Aldehyde and Ketone Mac-Jul 2013anessismanisNo ratings yet
- Alkyl Halides, Alcohols, Ethers and Epoxides: 1. What Is The IUPAC Name For CHDocument17 pagesAlkyl Halides, Alcohols, Ethers and Epoxides: 1. What Is The IUPAC Name For CHEllaŠtrbacNo ratings yet
- 09-Final With SolutionsDocument27 pages09-Final With SolutionsDanielle Wood100% (2)
- Chemistry AminesDocument8 pagesChemistry AminesVanshika LudhaniNo ratings yet
- Aldehydes, Ketones and Carboxylic AcidsDocument7 pagesAldehydes, Ketones and Carboxylic Acidskavitha2511977No ratings yet
- Diwali Assignment 12thDocument19 pagesDiwali Assignment 12thNishantPlayz YtNo ratings yet
- Organic SimplificationDocument13 pagesOrganic SimplificationKaushal Silva RanpatabendigeNo ratings yet
- M.SC 1st Sem Unit-2 2015Document6 pagesM.SC 1st Sem Unit-2 2015bgroyNo ratings yet
- SP 2007 Final Examination Organic II 200pts (Weighted As 300)Document21 pagesSP 2007 Final Examination Organic II 200pts (Weighted As 300)Ummi Khairani UrfaNo ratings yet
- Tutorial 3Document8 pagesTutorial 3Ahmad WahideeNo ratings yet
- Chemistry s5 Theory and Pract.Document29 pagesChemistry s5 Theory and Pract.ngabonzizayusuf9No ratings yet
- Graphene Oxide: Fundamentals and ApplicationsFrom EverandGraphene Oxide: Fundamentals and ApplicationsAyrat M. DimievNo ratings yet
- Arene Chemistry: Reaction Mechanisms and Methods for Aromatic CompoundsFrom EverandArene Chemistry: Reaction Mechanisms and Methods for Aromatic CompoundsNo ratings yet
- Chapter - 5dnonlinear Acoustic Propagation PDFDocument7 pagesChapter - 5dnonlinear Acoustic Propagation PDFAlinaBogoiNo ratings yet
- GE2023 Fundamentals of Nanoscience L T P C3 0 0 3 Unit I 9Document36 pagesGE2023 Fundamentals of Nanoscience L T P C3 0 0 3 Unit I 9yokeshNo ratings yet
- FYP List 2020 21RDocument3 pagesFYP List 2020 21RSaif UllahNo ratings yet
- Kinetics of A Particle Impulse and MomentumDocument17 pagesKinetics of A Particle Impulse and MomentumTejada, Brent LesterNo ratings yet
- 2 Methods of PolymerizationDocument13 pages2 Methods of Polymerizationbt21102047 Vishwajeet YadavNo ratings yet
- Narayana: Common Practice Test-04Document8 pagesNarayana: Common Practice Test-04narayana dwarkaNo ratings yet
- Baysolvex D2ehpa D2ehp eDocument4 pagesBaysolvex D2ehpa D2ehp evivianaramosmerinoNo ratings yet
- Hach Sample CellsDocument8 pagesHach Sample CellsKania LouisNo ratings yet
- Candidates Are Required To Give Their Answers in Their Own Words As Far As Practicable. The Figures in The Margin Indicate Full MarksDocument4 pagesCandidates Are Required To Give Their Answers in Their Own Words As Far As Practicable. The Figures in The Margin Indicate Full MarksAdhikari SushilNo ratings yet
- Electrochemical Energy-Part1Document59 pagesElectrochemical Energy-Part1GAYOSO, CHARLES F.No ratings yet
- 8-1 Worksheet PressureDocument2 pages8-1 Worksheet PressureGuillermo José Verbel MartínezNo ratings yet
- Workshet For Pre Board 1 XII 17-18Document4 pagesWorkshet For Pre Board 1 XII 17-18Sunita NinganurNo ratings yet
- RF Sputtering Manual 2010Document23 pagesRF Sputtering Manual 2010sinytells0% (1)
- Angels International CollegeDocument10 pagesAngels International CollegeTahira ZahidNo ratings yet
- Sulfur Slide ShowDocument14 pagesSulfur Slide ShowAbdulRehmanNo ratings yet
- Fiberline Screenroom Improvements and The Benefits in Pulp QualityDocument5 pagesFiberline Screenroom Improvements and The Benefits in Pulp QualityBastian Richard SitohangNo ratings yet
- Cahn-Ingold-Prelog Priority RulesDocument5 pagesCahn-Ingold-Prelog Priority RulesBer GuzNo ratings yet
- Multiphase Systems Containing A Component: Single CondensableDocument35 pagesMultiphase Systems Containing A Component: Single Condensableibrahim3318No ratings yet
- Bruce E. Depalma: N-Machine: Extraction of Electrical Energy Directly From Space: The N-MachineDocument7 pagesBruce E. Depalma: N-Machine: Extraction of Electrical Energy Directly From Space: The N-MachineHaris DizdarevicNo ratings yet
- Worksheet SAtoVratioworksheetwithkeyDocument2 pagesWorksheet SAtoVratioworksheetwithkeynim35No ratings yet
- Transient Heat Conduction: Course ContentsDocument13 pagesTransient Heat Conduction: Course ContentsJainil GajjarNo ratings yet
- F 2282 - 03 - RjiyodiDocument15 pagesF 2282 - 03 - RjiyodikrutikNo ratings yet
- Learning Unit 1 (Chap. 11)Document15 pagesLearning Unit 1 (Chap. 11)sbusisoNo ratings yet
- Electrical Circuits 1 1.1 Atomic Theory of MatterDocument12 pagesElectrical Circuits 1 1.1 Atomic Theory of MatterJohn Michael CabasaNo ratings yet
- 2021 JMRT EC RefinementDocument13 pages2021 JMRT EC Refinement김문조No ratings yet
- Energy Refractories Ammonia Methanol en 1004 TdsDocument2 pagesEnergy Refractories Ammonia Methanol en 1004 Tdsshuyang zhangNo ratings yet
- CH 31Document20 pagesCH 31Muzamil Shah100% (2)