Professional Documents
Culture Documents
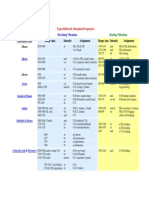
Table of IR Absorptions: Functional Group Characteristic Absorption(s) (CM Notes
Table of IR Absorptions: Functional Group Characteristic Absorption(s) (CM Notes
Uploaded by
aexmooOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Table of IR Absorptions: Functional Group Characteristic Absorption(s) (CM Notes
Table of IR Absorptions: Functional Group Characteristic Absorption(s) (CM Notes
Uploaded by
aexmooCopyright:
Available Formats
Table of IR Absorptions
Functional Group Characteristic Absorption(s)(cm1 ) 2950 - 2850 (m or s) Notes
Alkyl C-H Stretch
Alkane C-H bonds are fairly ubiquitous and therefore usually less useful in determining structure. Absorption peaks above 3000 cm-1 are frequently diagnostic of unsaturation
Alkenyl C-H Stretch Alkenyl C=C Stretch Alkynyl C-H Stretch Alkynyl C=C Stretch Aromatic C-H Stretch Aromatic C-H Bending Aromatic C=C Bending Alcohol/Phenol OH Stretch Carboxylic Acid OH Stretch
3100 - 3010 (m) 1680 - 1620 (v)
~3300 (s) 2260 - 2100 (v)
~3030 (v) 860 - 680 (s) 1700 - 1500 (m,m)
3550 - 3200 (broad, s) 3000 - 2500 (broad, v)
See "Free vs. Hyrdogen-Bonded Hydroxyl Groups" in the Introduction to IR Spectra for more information
Amine N-H Stretch
3500 - 3300 (m)
Primary amines produce two N-H stretch absorptions, secondary amides only one, and tetriary none.
Nitrile C=N Stretch Aldehyde C=O Stretch Ketone C=O Stretch Ester C=O Stretch
2260 - 2220 (m) 1740 - 1690 (s) 1750 - 1680 (s) 1750 - 1735 (s) 1780 - 1710 (s) 1690 - 1630 (s) The carbonyl stretching absorption is one of the strongest IR absorptions, and is very useful in structure determination as one can determine both the number of carbonyl groups (assuming peaks do not overlap) but also an estimation of which types.
Carboxylic Acid C=O Stretch Amide C=O Stretch Amide N-H Stretch 3700 - 3500 (m) As with amines, an amide produces zero to two N-H absorptions depending on its type.
All figures are for the typical case only -- signal positions and intensities may vary depending on the particular bond environment.
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Table of IR Absorptions: Functional Group Characteristic Absorption(s) NotesDocument2 pagesTable of IR Absorptions: Functional Group Characteristic Absorption(s) NotesMuhammad Khairuna SyahPutraNo ratings yet
- Table of IR Absorptions: Functional Group Characteristic Absorption(s) (CM NotesDocument2 pagesTable of IR Absorptions: Functional Group Characteristic Absorption(s) (CM NoteskeenakinkinNo ratings yet
- ASS Instrumental OrganicDocument17 pagesASS Instrumental OrganicMohamed SakrNo ratings yet
- Detailed Information On Carbonyl IRDocument3 pagesDetailed Information On Carbonyl IRtarhuniNo ratings yet
- Interpretation of Spectra of Different CompoundsDocument15 pagesInterpretation of Spectra of Different Compoundsmariam nawabNo ratings yet
- 6-IR Spectroscopy of Alkane, Alkene and Carbonyl CompoundsDocument8 pages6-IR Spectroscopy of Alkane, Alkene and Carbonyl Compoundsbloodhound13042005No ratings yet
- IR AbsorptionDocument3 pagesIR AbsorptionArtyNo ratings yet
- Functional Class Range (NM) Intensity Assignment Range (NM) Intensity AssignmentDocument6 pagesFunctional Class Range (NM) Intensity Assignment Range (NM) Intensity AssignmentdubstepoNo ratings yet
- IR Spectroscopy TutorialDocument36 pagesIR Spectroscopy TutorialreddygrNo ratings yet
- Infrared (IR) Spectroscopy: Structure, Purity, and IdentityDocument16 pagesInfrared (IR) Spectroscopy: Structure, Purity, and IdentityDiana KowsariNo ratings yet
- IR ProcedureDocument5 pagesIR ProcedureMuhammad FauziNo ratings yet
- Alcohol: Functional Group Type of Vibration Characteristic Absorptions (CM) IntensityDocument2 pagesAlcohol: Functional Group Type of Vibration Characteristic Absorptions (CM) IntensityMuhammad Fadhila Ragil YogaNo ratings yet
- IR-freq CO BondDocument3 pagesIR-freq CO BondRD's AcademyNo ratings yet
- Sample: Structure Functional Group Present Peaks (CM)Document2 pagesSample: Structure Functional Group Present Peaks (CM)MUHAMMAD USMANNo ratings yet
- Sample: Structure Functional Group Present Peaks (CM)Document2 pagesSample: Structure Functional Group Present Peaks (CM)MUHAMMAD USMANNo ratings yet
- Lecture 4 IR Spectrum AnalysisDocument43 pagesLecture 4 IR Spectrum AnalysiskhadijahhannahNo ratings yet
- DKO (Spektroskopi IR) : Arif Fadlan Gasal 2018/2019Document43 pagesDKO (Spektroskopi IR) : Arif Fadlan Gasal 2018/2019Hanan HakimNo ratings yet
- IR SpectrosDocument120 pagesIR Spectrosphd0780No ratings yet
- Raw Material Analysis-IRDocument58 pagesRaw Material Analysis-IRDilla Wulan NingrumNo ratings yet
- 424 Spectra TablesDocument19 pages424 Spectra TablespradeepiitdNo ratings yet
- 5 IR Analisis KualitatifDocument38 pages5 IR Analisis KualitatifPAHTMA PURNAMA SARI DEWINo ratings yet
- CHM391 - Practical Chemistry V Inorganic and AnalyticalDocument6 pagesCHM391 - Practical Chemistry V Inorganic and AnalyticalRejoice OdiaseNo ratings yet
- IRSpectrum AnalysisDocument2 pagesIRSpectrum AnalysisDavid S. FrohnapfelNo ratings yet
- Detailed Information On Carbonyl IRDocument2 pagesDetailed Information On Carbonyl IRismailhussain22No ratings yet
- Kuliah Infra Merah-LengkapDocument63 pagesKuliah Infra Merah-LengkapM Nur M. MahmudNo ratings yet
- Infrared SpectrosDocument110 pagesInfrared SpectrosBHARTI GAURNo ratings yet
- 2230L 08 IR Spectra InterpretationDocument11 pages2230L 08 IR Spectra Interpretationvennilaj23No ratings yet
- Spektro IRDocument64 pagesSpektro IRAnonymous NSK4nvH4ufNo ratings yet
- Spec Ir NMR Spectra TablesDocument15 pagesSpec Ir NMR Spectra TablesMah NovaesNo ratings yet
- IR SPECTROSCOPY Notes FullDocument5 pagesIR SPECTROSCOPY Notes FullKartik KuteNo ratings yet
- Aplikasi Infra Merah Dalam Bidang Farmasi: Abdul Rohman Laboratorium Kimia Analisis Fakultas Farmasi UGMDocument38 pagesAplikasi Infra Merah Dalam Bidang Farmasi: Abdul Rohman Laboratorium Kimia Analisis Fakultas Farmasi UGMKhumairah MohtarNo ratings yet
- BÀI TẬP MẪUDocument13 pagesBÀI TẬP MẪUMinh Tân LêNo ratings yet
- Spectroscopy Tutorial: Reference: Wavenumber, cm-1 Bond Functional GroupDocument2 pagesSpectroscopy Tutorial: Reference: Wavenumber, cm-1 Bond Functional Groupnaneunlaila_44237576No ratings yet
- Infrared Tutorial 2Document71 pagesInfrared Tutorial 2Hammo Ez AldienNo ratings yet
- IR Analisis KualitatifDocument38 pagesIR Analisis KualitatifRetno SulistyaningrumNo ratings yet
- Infrared Spectroscopy: IR Absorptions For Representative Functional GroupsDocument3 pagesInfrared Spectroscopy: IR Absorptions For Representative Functional GroupsSaleem BashaNo ratings yet
- Ir Func GroupDocument52 pagesIr Func GroupEry NourikaNo ratings yet
- Introduction To Interpretation of Infrared SpectraDocument3 pagesIntroduction To Interpretation of Infrared SpectraBenni WewokNo ratings yet
- Characteristic Infrared Absorption FrequenciesDocument1 pageCharacteristic Infrared Absorption FrequenciesVirendra Singh RajputNo ratings yet
- Aplikasi Spek IR Untuk FarmasiDocument38 pagesAplikasi Spek IR Untuk FarmasiHifdzurRashifRija'iNo ratings yet
- Solomons Organic Chemistry Module IR TableDocument1 pageSolomons Organic Chemistry Module IR TableBenni WewokNo ratings yet
- IR Analysis: Characteristic Infrared Absorption Frequencies Bond Compound Type Frequency Range, CMDocument1 pageIR Analysis: Characteristic Infrared Absorption Frequencies Bond Compound Type Frequency Range, CMaamer_shahbaazNo ratings yet
- CHMBD 449 - Organic Spectral: AnalysisDocument43 pagesCHMBD 449 - Organic Spectral: AnalysisIleana ManciuleaNo ratings yet
- Table of Characteristic IR AbsorptionsDocument4 pagesTable of Characteristic IR Absorptionsأمالي أريفينNo ratings yet
- Loudon 5 Ech 12 Sec 04Document6 pagesLoudon 5 Ech 12 Sec 04Amylase NguyenNo ratings yet
- Aplikasi Infra Merah Dalam Bidang Farmasi: Muhammad Taupik Laboratorium Kimia Analisis Farmasi UNGDocument35 pagesAplikasi Infra Merah Dalam Bidang Farmasi: Muhammad Taupik Laboratorium Kimia Analisis Farmasi UNGVsten MramisNo ratings yet
- Applications of Infra Red SpectrosDocument5 pagesApplications of Infra Red SpectrosEditor IJTSRDNo ratings yet
- MASS SPECTROMETRY Only Fragmentation Pattern - OCT.2024Document23 pagesMASS SPECTROMETRY Only Fragmentation Pattern - OCT.2024aimaananwarNo ratings yet
- Experiment 3 chm260 ManDocument11 pagesExperiment 3 chm260 ManarissaNo ratings yet
- IR SpectrosDocument33 pagesIR SpectrosKikiMariaNo ratings yet
- B.Tech Spectroscopy Module 5.4: Applications of IR SpectrosDocument16 pagesB.Tech Spectroscopy Module 5.4: Applications of IR SpectrosKAMALANo ratings yet
- IR Spectra AnalysisDocument37 pagesIR Spectra AnalysisdevoydouglasNo ratings yet
- IR Absorption Table PDFDocument3 pagesIR Absorption Table PDFDavid QuinteroNo ratings yet
- Interpretation of IR Compounds BY KhanDocument7 pagesInterpretation of IR Compounds BY KhanShamsheer KhanNo ratings yet
- BP701T Ima IiDocument36 pagesBP701T Ima Iidwivedishrishti18No ratings yet
- IR Spectroscopy (Solutions)Document4 pagesIR Spectroscopy (Solutions)LakshayNo ratings yet
- Images from Lichenes Australasici Exsiccati and of other characteristic Australasian Lichens. Volume OneFrom EverandImages from Lichenes Australasici Exsiccati and of other characteristic Australasian Lichens. Volume OneNo ratings yet