Professional Documents
Culture Documents
Energy States of Chemical Species: Two Important Postulates of Quantum Theory
Energy States of Chemical Species: Two Important Postulates of Quantum Theory
Uploaded by
Subhash DhungelOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Energy States of Chemical Species: Two Important Postulates of Quantum Theory
Energy States of Chemical Species: Two Important Postulates of Quantum Theory
Uploaded by
Subhash DhungelCopyright:
Available Formats
Energy States of Chemical Species
-Quantum theory was first proposed by Max Planck in 1900. -It was to explain the properties of radiation emitted by heated bodies. -Later extended to rationalize other types of emission and absorption processes.
Two important postulates of quantum theory:
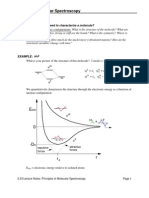
a)Atoms, ions, and molecules can exist only in certain certain discrete energy states. b)To change the states it absorbs or emits an amount of energy exactly equal to the energy difference between the states. i.e. Higher energy state(E1) Lower(E0) = h= hc Where, c is velocity of light, h is Plank constant (6.6254x10-34) & is wavelength. -Energy of the elemental states(atoms,ions) is attributed to the motion of electrons around the positively charged nucleus. (Electronic states) -In case of a molecule, in addition to translational motion & it electronic energy states possesses other internal energy in their quantised states. These source of energy can be subdivided into three classes: i)Rotational energy: Associated with the rotation of the molecule about an axis (Rotational state) ii)Vibrational energy: (Vibrational state) Associated with movement of the atoms or groups with respect to each other(periodic movement)
iii)Electronic energy: (Electronic state)
Associated with electronic configuration and electronic state of the molecule
So that, total internal energy states of molecules: Eint = Eelect. + Evib. + Erot. -The permitted energy levels of Eelect.,Evib. & Erot. are intimately related to the structure of the molecule.
-No two kinds of molecules possess identical Eelect.,Evib. & Erot. -Ground state: The lowest energy state of an atom or molecule.(generally at room Temp.) -Excited state: Higher energy states.
You might also like
- Charles W. Lucas, JR - A New Foundation For Modern PhysicsDocument12 pagesCharles W. Lucas, JR - A New Foundation For Modern PhysicsJuiomSDFNo ratings yet
- Transformation of SentencesDocument11 pagesTransformation of SentencesSubhash Dhungel100% (8)
- Quantum Theory and Atomic StructureDocument52 pagesQuantum Theory and Atomic StructureBeri NaysNo ratings yet
- Physical Chemistry FaderuDocument76 pagesPhysical Chemistry FaderumikinaniNo ratings yet
- Electronic Structure of Atoms - General ChemistryDocument92 pagesElectronic Structure of Atoms - General ChemistryDuc Anh NguyenNo ratings yet
- CHAPTER # 1the Nature of Matter and Periodicity of Atomic PropertiesDocument48 pagesCHAPTER # 1the Nature of Matter and Periodicity of Atomic PropertiesAnonymous UfzcLV8ZNo ratings yet
- Bohr's Atomic Model L: Stationary OrbitsDocument2 pagesBohr's Atomic Model L: Stationary OrbitsPalash KhareNo ratings yet
- Bohrs Atomic and The HistoryDocument14 pagesBohrs Atomic and The HistoryNurul Assikin Binti AriffinNo ratings yet
- WEEK 4 CDocument13 pagesWEEK 4 CeodurotwumNo ratings yet
- Module 1 NotesDocument32 pagesModule 1 NotesjoelleNo ratings yet
- Electronic Structure of AtomsDocument59 pagesElectronic Structure of Atomstalktotiffanycheng100% (1)
- Chaper 5Document41 pagesChaper 5gebrewahidbiniam75No ratings yet
- Principles of Molecular SpectrosDocument12 pagesPrinciples of Molecular SpectrosShaheentariq_4No ratings yet
- CHM012 - Module 3 (Part 2)Document16 pagesCHM012 - Module 3 (Part 2)haibaalisa00No ratings yet
- Structure of Atoms - NotesDocument8 pagesStructure of Atoms - NotesShrey parikhNo ratings yet
- PHYSICSDocument1 pagePHYSICSzoya last nameNo ratings yet
- Atomic Model NotesDocument5 pagesAtomic Model Notesoyamo markNo ratings yet
- CH 12 NotesDocument2 pagesCH 12 NotesAila Kublai-sikNo ratings yet
- Chapter 2Document12 pagesChapter 2joseNo ratings yet
- Quantum Lecture CML 1Document53 pagesQuantum Lecture CML 1samiNo ratings yet
- Atoms Truc TDocument9 pagesAtoms Truc Tpavi32No ratings yet
- Different Atom ModelsDocument13 pagesDifferent Atom ModelsNoor Mohammad NofaerNo ratings yet
- CH B1. Summary-Models of The Atom WRKSHTDocument3 pagesCH B1. Summary-Models of The Atom WRKSHTellamocsNo ratings yet
- CH 2 The Particle Properties of WavesDocument21 pagesCH 2 The Particle Properties of Wavesyohanse mehabawNo ratings yet
- Ecture O.: (The Electronic Structure of Atoms)Document43 pagesEcture O.: (The Electronic Structure of Atoms)Yarisse RivasNo ratings yet
- SRM Institute of Science and Technology, Ramapuram Semiconductor Physics (18PYB103J) Unit - IDocument32 pagesSRM Institute of Science and Technology, Ramapuram Semiconductor Physics (18PYB103J) Unit - IRN DpkNo ratings yet
- A Modern Course in The Quantum Theory of Solids Capitulo 1Document42 pagesA Modern Course in The Quantum Theory of Solids Capitulo 1Baco DavisNo ratings yet
- Quantum Theory and The Electronic Structure of AtomsDocument17 pagesQuantum Theory and The Electronic Structure of AtomsSalama NaumanNo ratings yet
- Chapter 11,12,13 Test Paragraph Question PaperDocument2 pagesChapter 11,12,13 Test Paragraph Question PapershiviNo ratings yet
- HODANDocument6 pagesHODANSuleymaan AhmedNo ratings yet
- Atomic StrucutreDocument41 pagesAtomic Strucutreganeshdhembare89No ratings yet
- 2016 Bookmatter TheQuantumHandshakeDocument48 pages2016 Bookmatter TheQuantumHandshakeAkshay SatputeNo ratings yet
- Science Behind Atomic BombDocument8 pagesScience Behind Atomic BombWaqar AsgharNo ratings yet
- Contemporary Global IssuesDocument15 pagesContemporary Global IssuesBisrateab FekaduNo ratings yet
- The Bohr AtomDocument5 pagesThe Bohr AtomHayley WelshNo ratings yet
- Quantum Numbers: Introduction To ChemistryDocument24 pagesQuantum Numbers: Introduction To Chemistryhimanshu yadavNo ratings yet
- School WorkDocument15 pagesSchool Workjdubey4258No ratings yet
- Molecular Orbital Theory: Luis Bonilla Abel Perez University of Texas at El Paso Molecular Electronics, Chem 5369Document26 pagesMolecular Orbital Theory: Luis Bonilla Abel Perez University of Texas at El Paso Molecular Electronics, Chem 5369viraivil9417No ratings yet
- Ch1 Revision of EP203Document31 pagesCh1 Revision of EP203mirckyNo ratings yet
- Elementary Concepts of Material Science: HapterDocument23 pagesElementary Concepts of Material Science: Haptersuccess_tusharNo ratings yet
- Concept MapDocument3 pagesConcept MapAlyssa BihagNo ratings yet
- Ass Mahfuz Sir Byb SiamDocument10 pagesAss Mahfuz Sir Byb SiamNowfal Hasan SiamNo ratings yet
- Elements of Physical Chemistry Atkins & DepaulaDocument29 pagesElements of Physical Chemistry Atkins & DepaulaSub mNo ratings yet
- Ilrds 1Document49 pagesIlrds 1ELLAINE MAE FLORESNo ratings yet
- Modern PhysicsDocument15 pagesModern Physicsabdogh93No ratings yet
- Molecular Energy Levels: DR Imrana AshrafDocument50 pagesMolecular Energy Levels: DR Imrana AshrafKashif Rehman MalikNo ratings yet
- Network Analysis Chapter 1 - Mac E. Van ValkenburgDocument28 pagesNetwork Analysis Chapter 1 - Mac E. Van Valkenburglindatrummer5550% (2)
- Notes - Quantum Theory of Light and Matter WavesDocument34 pagesNotes - Quantum Theory of Light and Matter Wavesphysics olympiad100% (1)
- CBSE Class 11 Chemistry Notes CH - 02 Structure of AtomDocument6 pagesCBSE Class 11 Chemistry Notes CH - 02 Structure of AtomVivek Saahil40% (5)
- X RayDocument12 pagesX RayNeetin AgrawalNo ratings yet
- Science Reviewer 2nd QTRDocument23 pagesScience Reviewer 2nd QTREl GamezNo ratings yet
- Fisica Moderna PonceDocument74 pagesFisica Moderna PonceWilmer MoncadaNo ratings yet
- P 62Document25 pagesP 62JohnnardBelenNo ratings yet
- Cape Chemistry Unit 1 Module 1 Notes 2023Document30 pagesCape Chemistry Unit 1 Module 1 Notes 2023chelsea AlexandriaNo ratings yet
- Quantum MechanicsDocument46 pagesQuantum MechanicsAbhilash Nair50% (2)
- Assignment 1Document7 pagesAssignment 1Noor ul HudaNo ratings yet
- Quantum Mechanics PyEd 342Document113 pagesQuantum Mechanics PyEd 342mesfint100% (3)
- KOE043Document15 pagesKOE043Utkarsh KumarNo ratings yet
- Tutorial 28, Planks Quantum Theory, Duel Nature of LightDocument9 pagesTutorial 28, Planks Quantum Theory, Duel Nature of LightDYES Motion GraphicsNo ratings yet
- What is Charge? – The Redefinition of Atom - Energy to Matter ConversionFrom EverandWhat is Charge? – The Redefinition of Atom - Energy to Matter ConversionNo ratings yet
- AnalysisDocument4 pagesAnalysisSubhash DhungelNo ratings yet
- LiquidDocument7 pagesLiquidSubhash DhungelNo ratings yet
- Experiments On Gramimetry and Precipitation TitrationDocument4 pagesExperiments On Gramimetry and Precipitation TitrationSubhash DhungelNo ratings yet
- Biological AssaysDocument19 pagesBiological AssaysSubhash DhungelNo ratings yet
- How To Do A Patient Follow-UpDocument3 pagesHow To Do A Patient Follow-UpSubhash DhungelNo ratings yet
- Assay of Acetyl Salicylic Acid and Limit of Salicylic Acid.: Title:Quality Assurance of Aspirin Tablets On The Basis ofDocument4 pagesAssay of Acetyl Salicylic Acid and Limit of Salicylic Acid.: Title:Quality Assurance of Aspirin Tablets On The Basis ofSubhash DhungelNo ratings yet
- Mathematical Description of A WaveDocument3 pagesMathematical Description of A WaveSubhash DhungelNo ratings yet
- Absorption Methods: I) Transmittance (T)Document2 pagesAbsorption Methods: I) Transmittance (T)Subhash DhungelNo ratings yet
- Superposition of WavesDocument3 pagesSuperposition of WavesSubhash DhungelNo ratings yet
- Quantum Mechanical ConceptDocument4 pagesQuantum Mechanical ConceptSubhash DhungelNo ratings yet
- ErrorsDocument6 pagesErrorsSubhash DhungelNo ratings yet
- Diffraction of RadiationDocument3 pagesDiffraction of RadiationSubhash DhungelNo ratings yet
- Laser Sources:: ComponentsDocument5 pagesLaser Sources:: ComponentsSubhash DhungelNo ratings yet
- Sources of Coherent RadiationDocument7 pagesSources of Coherent RadiationSubhash DhungelNo ratings yet
- Quantum Mechanical ConceptDocument4 pagesQuantum Mechanical ConceptSubhash DhungelNo ratings yet
- Analytical MethodsDocument16 pagesAnalytical MethodsSubhash Dhungel100% (1)