Professional Documents
Culture Documents
PK - FKK.PPM - Manual Makmal Che565 (0) : Chemical Engineering Laboratory Iii
PK - FKK.PPM - Manual Makmal Che565 (0) : Chemical Engineering Laboratory Iii
Uploaded by
mnizamarzukiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
PK - FKK.PPM - Manual Makmal Che565 (0) : Chemical Engineering Laboratory Iii
PK - FKK.PPM - Manual Makmal Che565 (0) : Chemical Engineering Laboratory Iii
Uploaded by
mnizamarzukiCopyright:
Available Formats
UNIVERSITI TEKNOLOGI MARA
FAKULTI KEJURUTERAAN KIMA
CHEMICAL ENGINEERING LABORATORY III
(CHE575)
NAME : RM SYIBLI MILASI B R MUHAMAD FAKIH
STUDENT NO. : 200424!2!
E"#ERIMENT : CONTINUOUS STIRRED TANK REACTOR (CSTR) IN SERIES
DATE PERFORMED : 7
TH
FEBUARY 200
SEMESTER :
DISEMBER 2005 APRIL 2006
#ROGRAMME $ CODE :
Bachelor of Engineering (Hons. in !he"ical Engineering
$ EH220
Remarks:
Checked by: Rechecked by:
DR.RUZITAH
No. Ti!e A!!ocaed marks " Marks "
# Absrac$%&mmary '
( I)rod&cio) '
* Aims$Ob+eci,es '
- Theory '
' Proced&res *
. A//ara&s '
0 Res&!s (1
2 Ca!c&!aio)s #1
3 Disc&ssio)s (1
#1 Co)c!&sio)s #1
## Recomme)daio)s '
#( Re4ere)ces '
#* A//e)dices (
TOTA5 #11
P6.F66.PPM.MANUA5 MA6MA5 CHE'.'
718
TABLE OF CONTENTS
ABSTRACT$SUMMARY99999999999.9.*
INTRODUCTION9999999999999.........*:-
OBJECTIVES99999999999999999-
THEORY99999999999999999......-:2
#ROCEDURES999999999999999..2:#1
A##ARATUS99999999999999999.#1
RESULTS99999999999999999##:#(
SAM#LE OF CALCULATIONS999999999#*
DISCUSSION999999999999999.#-:#'
CONCLUSION9999999999999999.#'
RECOMMENDATION9999999999999.#.
REFERENCES9999999999999999.#.
A##ENDICES9999999999999999...#0
S#MMAR$
Our experiment involves a continuous stirred tank reactor (CSTR) in series. Our
system consists of 3 agitated, glass reactor vessels in series. lt!oug! t!e concentration is
uniform for eac! reactor "ut t!ere is a c!ange in concentration as fluids move over from
reactor to reactor.
Our o"#ective in t!is experiment is to determine t!e concentration response to a
step c!ange and pulse input and also to determine t!e effect of residence time on t!e
response curve.
$
st
t!e deionised %ater are filled in t!e "ot! t%o tanks %it! t!e sodium c!loride
%ere diluted in t!e tank one. T!en deionised %ater from t!e tank t%o %ill flo% t!roug! to
fill up t!e t!ree reactors. T!e flo% rate of t!e deionised %ater is set to $&' ml(min to
prevent from over flo%. T!e only readings %ere taken at time t
o
after %e get t!e readings
of t!e conductivity are sta"le enoug! %!ere t!e readings of t!e conductivity are )uit
similar from one to anot!er. fter t!at, readings are continuously taken every 3 minutes
until to t!e point t!at t!e conductivity values for t!e t!ree reactors are closed to eac!
ot!er. T!en t!e grap! of t!e conductivity versus time %as plotted
T!e grap! t!at !as "een plotted is accordingly to t!e t!eory. *rom t!e grap! %e
can determine t!e effect of t!e step c!ange and pulse input to t!e concentration.
I%&R'D#!&I'%
+n t!e ma#ority of industrial c!emical process, a reactor is t!e key item of
e)uipment in %!ic! ra% materials undergo a c!emical c!ange to form desired product.
T!e design and operation of c!emical reactors is t!us crucial to t!e %!ole success of t!e
industrial operation.
Reactors can %idely form, depending on t!e nature of t!e feed materials and t!e
products. ,nderstanding non-steady "e!avior of process e)uipment is necessary for
design and operation of automatic control systems. One particular type of process
e)uipment is t!e continuous stirred tank reactor. +n t!is reactor, it is important to
determine t!e system response to a c!ange in concentration. T!is response of
concentration versus time is an indication of t!e ideality of t!e system.
'B(E!&I)ES
$. To determine t!e effect of step c!anges and pulse input to t!e concentration.
.. To determine t!e effect residence time on t!e response curve.
&HE'R$
;e)era! Mo!e <a!a)ce E=&aio)
Ass&m/io)s
#8 %eady sae here4ore
(8 >e!! mi?ed here4ore rA is he same hro&@ho& he reacor
Rearra)@i)@ he @e)eraio)
I) erms o4 co),ersio)
Reacors i) %eries
;i,e) :rA as a 4&)cio) o4 co),ersio)A A :rA B 47C8A o)e ca) a!so desi@) a)y
se=&e)ce o4 reacors i) series /ro,ided here are )o side sreams by de4i)i)@ he
o,era!! co),ersio) a a)y /oi).
Mo!e <a!a)ce o) Reacor #
Mo!e <a!a)ce o) Reacor (
;i,e) :rA B 47C8 he 5e,e)s/ie! P!o ca) be &sed o 4i)d he reacor ,o!&me
For a PFR beDee) Do C%TRs
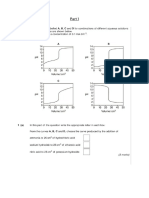
E44ec o4 %e/ Cha)@e i) I)/& Co)ce)raio) o he Co)ce)raio) o4 %o!&e i)
%irred Ta)k Reacors i) %eries.
>he) a se/ cha)@e o4 so!&e co)ce)raio) is i)rod&ced a he 4eed o4 a)k #A
he a)k i) series Di!! e?/erie)ce a ra)sie) beha,ior as a Fi@&re 0 be!oD. The
res/o)se Di!! be de/e)de) o) he reside)ce ime o4 each reacor i) series.
Co)ce)raio) Co)ce)raio)
::::::::::::::::::::::::::::::::::
Time Time
Fi@&re 2a. %e/ cha)@e i)/& Fi@&re 2b . Tra)sie) res/o)se o4
a)k i) series o he se/ i)/&.
E44ec o4 P&!se i) I)/& Co)ce)raio) o he Co)ce)raio) o4 %o!&e i) %irred
Ta)k i) %eries.
Reacor #
Reacor (
Reacor *
R%&'()* (
R%&'()* +
R%&'()* *
>he) a /&!se i)/& o4 so!&e co)ce)raio) is i)rod&ced a he 4eed o4 a)k #A he
ra)sie) beha,ior Di!! be di44ere) ha) he se/ cha)@e i)/& d&e o he
dimi)ishi)@ co)ce)raio) 4rom he i)/& a4er /&!si)@ as described i) Fi@&re 2.
T,-% T,-%
F,./*% !&: #/01% ,23/( F,./*% !4: T*&21,%2( *%13)21% )5
(&26 ,2 1%*,%1 () (7% 3/01% ,23/(.
PR'!ED#RES
E*+eri"en, -. &he Effec, of S,e+ !hange In+/,
+n t!is experiment a step-c!ange input %ould "e introduced and t!e progression of t!e
tracer %ill "e monitored via t!e conductivity measurements in all t!e t!ree reactors.
Tank $ and tank . %as filled up %it! .'/ feeds deionised %ater.
3''g of Sodium C!loride %as dissolved in tank $until t!e salts dissolve entirely
and t!e solution is !omogenous.
T!ree %ay valve (03) %as set to position . so t!at deionised %ater from tank .
%ill flo% into reactor $.
1ump . %as s%itc!ed on to fill up all t!ree reactors %it! deionised %ater.
T!e flo% rate (*l$) %as set to $&' ml(min "y ad#usting t!e needles valve (02). 3o
C
)
2
'
%
2
(
*
&
(
,
)
2
C
)
2
'
%
2
(
*
&
(
,
)
2
not use too !ig! flo% rate to avoid t!e over flo% and make sure no air "u""les
trapped in t!e piping. T!e stirrers $, . and 3 %ere s%itc!ed on.
T!e deionised %ater %as continued pumped for a"out $' minute until t!e
conductivity readings for all t!ree reactors %ere sta"le at lo% values.
T!e values of conductivity %ere recorded at t
'
.
T!e pump . %as s%itc!ed off after & minutes. T!e valve (03) %as s%itc!ed to
position $ and t!e pump $ %as s%itc!ed on. T!e timer %as started.
T!e conductivity values for eac! reactor %ere recorded every t!ree minutes.
Record t!e conductivity values %ere continued until reading for reactor 3 closed
to reactor $.
1ump . %as s%itc!ed off and t!e valve (02) %as closed.
ll li)uids in reactors %ere drained "y opening valves 0& and 04.
E*+eri"en, 2. &he Effec, of P/lse In+/,
+n t!is experiment a pulse input %ould "e introduced and t!e progression of t!e tracer
%ill "e monitored via t!e conductivity measurements in all t!e t!ree reactors.
Tank $ and tank . %as filled up %it! .'/ feeds deionised %ater.
3''g of Sodium C!loride %as dissolved in tank $until t!e salts dissolve entirely
and t!e solution is !omogenous.
T!ree %ay valve (03) %as set to position . so t!at deionised %ater from tank .
%ill flo% into reactor $.
1ump . %as s%itc!ed on to fill up all t!ree reactors %it! deionised %ater.
T!e flo% rate (*l$) %as set to $&' ml(min "y ad#usting t!e needles valve (02). 3o
not use too !ig! flo% rate to avoid t!e over flo% and make sure no air "u""les
trapped in t!e piping. T!e stirrers $, . and 3 %ere s%itc!ed on.
T!e deionised %ater %as continued pumped for a"out $' minute until t!e
conductivity readings for all t!ree reactors %ere sta"le at lo% values.
T!e values of conductivity %ere recorded at t
'
.
T!e pump . %as s%itc!ed off after & minutes. T!e valve (03) %as s%itc!ed to
position $ and t!e pump $ %as s%itc!ed on. T!e timer %as started.
/et t!e pump $ to operate for & minute, and t!en s%itc!ed off pump $. S%itc!ed
t!e t!ree %ays valve (03) "ack to position .. T!e pump . %as s%itc!ed on.
T!e conductivity values for eac! reactor %ere recorded every t!ree minutes.
Record t!e conductivity values %ere continued until reading for reactor 3 closed
to reactor $.
1ump . %as s%itc!ed off and t!e valve (02) %as closed.
ll li)uids in reactors %ere drained "y opening valves 0& and 04.
APPARA&#S
#. Disi!!aio) Daer
(. %odi&m Ch!oride
*. Co)i)&o&s reacor i) series
-. %irrer sysem
'. Feed a)ks
.. >ase a)k
0. Dead ime coi!
2. Com/&eriEe sysem
3. %o/ Dach
CO5T+5,O,S/6 ST+RR73 T58 R7CTOR (CSTR)
SAMPLE '0 !AL!#LA&I'%
0
i
9 *
'
(:
,i
- :
,i-$
) ( (-r
)
i
;!ere 0i 9 volume of reactor i
*
,i
9 molal flo% rate of into t!e first reactor
:
,i
9 fractional conversion of in t!e reactor i
:
,i-$
9 fractional conversion of in t!e reactor i-$
*or first order reaction, -r
9 k C
,+
9 kC
'
($ < :
,i
)
v 9 volumetric flo% rate of 9 $&'mil(min 9 '.$& liter(min
*or t!e first reactor= (0 9 .' lit)
(-r
)$ 9 (kC
)
$
9 kC
,$
9 k C
'
($ < :
,$
)
C
'
9 *
'
( v
i.e., *
'
9 vC
'
:
,i-$
9 :
,'
9 '
T!erefore,
Tank $
0i 9 *
'
(:
,i
< :
,i-$
) ( (-r
)
i
.' 9 '.$& (:
,$
< ') ( ('.$&> x ($ < :
,$
))
:
,$
9 '.?&
Tank .
0i 9 *
'
(:
,i
< :
,i-$
) ( (-r
)
i
.' 9 '.$& (:
,$
< '.?&) ( ('.$&> x ($ < :
,$
))
:
,$
9 '.??@
Tank 3
0i 9 *
'
(:
,i
< :
,i-$
) ( (-r
)
i
.' 9 '.$& (:
,$
< '.??@) ( ('.$&> x ($ < :
,$
))
:
,$
9 '.??>
Disc/ssion
+n t!is experiment our o"#ective is to determine t!e effect of step c!ange and
pulse input to t!e concentration in a continues stirred tank reactor in series. *or t!at %e
!ave . experiment $
st
t!e step c!ange and .
nd
is t!e pulse input.
;e take t!e reading of t!e conductivity for t!e 3 different tanks for every 3
minutes and plotted grap! conductivity versus time. *or t!e $
st
experiment t!e flo% rate
%as $&' ml(min and %e take .> readings. ;e can see t!e effect of t!e step c!ange to t!e
concentration from t!e grap!. Step c!ange is a sudden c!ange in a process varia"le. *or
t!is experiment our varia"le t!at "een c!ange is t!e input. Reactor feedstock is suddenly
s%itc!ed from one supply to anot!er, causing sudden c!anges in feed concentration,
flo%, etc.
s %e kno% t!e concentration can "e calculated using electrical conductivity
measurements and cali"ration supplied. T!e concentration is directly proportional to t!e
conductivity.
T!e affect of t!e step c!ange to t!e concentration for t!e 3 reactors are t!e same.
;!en t!e step c!ange of solute concentration %as introduced at t!e feed of tank $, t!e
tank %ill experience a transient "e!avior as s!o%n in t!e result. T!e concentration of in
t!e reactor %ill increase in a period of time until it !as reac!ed a constant concentration.
*or every reactor it !as its o%n concentration. Reactor $ !as t!e !ig!est concentration
follo%ing tank . and 3. T!e concentration if increasing "ecause of t!e feed rate t!at "een
opened %as t!e tank $ t!at contain t!e dissolve c!loride.
*or t!e .
nd
experiment t!e procedure is t!e same as experiment $ except %e
c!ange t!e pump from $ to . and t!e flo% rate %as .''mil(min. *or t!is experiment %e
%ant to see t!e effect of t!e pulse input. ;!en %e !ave plotted t!e grap! %e get a
different result from t!e step c!ange. T!e pulse input caused t!e concentration c!ange
drastically for every tank. *or tank $ t!e concentration decreased rapidly until it reac!es
a constant value. *or tank . t!e concentrations $st increase in a period of time t!en start
decreasing until it reac!es a constant value. *or tank 3 t!e concentrations also increase
and decrease "ut not rapidly. T!ere are only a small different for every readings.
T!e decreasing in t!e concentration !appen "ecause t!e feed %as from tank . t!at
contain only dissolve %ater %it!out any sodium c!loride.
!oncl/sions
*rom t!e experiment result it s!o%s t!at a step c!ange in input and a pulse in
input !ave its o%n effect to t!e concentration. 7ac! of t!is experiment !as its o%n
transient "e!avior. ;e compare t!e $
st
grap! and t!e .
nd
grap!.
*or t!e $
st
grap! t!e c!ange of t!e concentration for t!e 3 tank is almost t!e same.
*or t!e .
nd
grap! every tank !as its o%n c!ange of concentration. *rom "ot! experiment
%e can conclude t!at c!ange in input and pulse input !as an effect to t!e concentration.
*or t!e step c!ange it %ill increase t!e concentration until it reac!es a constant value and
for pulse input it %ill first increase t!en decrease until it reac!es a constant value. T!e
feed of t!e systems effect t!e concentration in t!e reactor, if t!e feed contain a
concentration t!en t!e concentration in t!e tank %ill increase and if t!e feed only contain
deionised %ater t!en t!e concentration %ill decrease.
7very reactor !as its o%n concentration, "ecause of t!at %e conclude t!at t!e
residence time for eac! reactor is different. T!e value of t!e residence time depends on
%!at !appens in t!e reactor.
Reco""en1a,ions
fter %e !ave finis!ed t!is experiment, %e find t!at are several factors in t!is
experiment t!at can "e fixed to make sure t!at t!e experiment runs "etter. T!is is some of
my recommendation for t!is experiment=
;!en %e are doing t!e experiment t!e program t!at used to record t!e data %as
not function. T!is cause us a !ig! error in reading t!e data. Ay recommendation
is to make sure "etter maintainers of t!e apparatus.
T!e instruction in t!e la" manual for num"er $,. and 3 for "ot! experiment is not
clear and after %e doing t!e experiment it seems t!at %e did not follo% t!e
procedures $,. and 3. ;e #ust #ump to step num"er 2, it cause us a %aste of time
and confusion. Ay recommendation is to make sure t!at t!e procedure is exactly
t!e same as %!at %e do in t!e experiment.
Reference
5e,e)s/ie!A OA Chemical Reaction EngineeringA Foh) >i!eyA #30(
Rober H.PerryA Do) >.;ree)A Perrys Chemical Engineers HandbookA
Mc;raD Hi!!A#332.
%mihAF.MA Chemical Engineering Kinetics, Mc;raD Hi!!A #32#.
A++en1ics
C1 C2
C3
Fi@&re '
T83% )5 R%&'()* C7&*&'(%*,1(,'1
Co)i)&o&s!y %irred
Ta)k Reacor 7C%TR8
R&) a seady sae Dih co)i)&o&s 4!oD o4 reaca)s a)d
/rod&csG he 4eed ass&mes a uniform com/osiio)
hro&@ho& he reacorA exit sream has he same com/osiio)
as i) he a)k
K,291 )5
#7&1%1
#*%1%2(
U1&.% A9:&2(&.%1 D,1&9:&2(&.%1
#. 5i=&id /hase
(. ;as:!i=&id
r?)s
*. %o!id:!i=&id
r?)s
#. >he) a@iaio) is
re=&ired
(. %eries
co)4i@&raio)s 4or
di44ere)
co)ce)raio)
sreams
#. Co)i)&o&s o/eraio)
(. ;ood temperature
control
*. Easi!y ada/s o Do
/hase r&)s
-. ;ood co)ro!
'. %im/!iciy o4
construction
. 5oD o/erai)@ 7!abor8
cos
#. 5oDes co),ersio)
/er &)i ,o!&me
(. <y:/assi)@ a)d
cha))e!i)@ /ossib!e
Dih /oor a@iaio)
You might also like
- Data Tables For Alien Gene AnalysisDocument3 pagesData Tables For Alien Gene Analysisapi-382372564100% (3)
- CSTR Lab ReportDocument16 pagesCSTR Lab Reportleenzalal100% (5)
- CSTR Lab Report .Document18 pagesCSTR Lab Report .Emily Swan50% (4)
- Continuous Distillation ColumnDocument31 pagesContinuous Distillation ColumnRichard Obinna100% (2)
- MCAT Prep Organic Equation SheetDocument6 pagesMCAT Prep Organic Equation SheetChris_Barber09No ratings yet
- Laboratory ReportDocument31 pagesLaboratory ReportJim100% (2)
- Title Page: The Total Mass Flow Rate of A SystemDocument35 pagesTitle Page: The Total Mass Flow Rate of A SystemJimNo ratings yet
- Lab Report CSTR 40LDocument26 pagesLab Report CSTR 40LAnonymous NyvKBW33% (3)
- Lab CSTR in SeriesDocument13 pagesLab CSTR in SeriesKhairul Zakirin78% (9)
- Experiment 1 CSTR DynamicsDocument24 pagesExperiment 1 CSTR DynamicsFarhan Hazeeq50% (2)
- Lab Report CSTR in SeriesDocument13 pagesLab Report CSTR in SeriesNisha Sharif100% (1)
- CSTR Lab ReportDocument10 pagesCSTR Lab ReportErraFatihaNo ratings yet
- Lab Report Batch Reactor GGDocument25 pagesLab Report Batch Reactor GGFrost Orchid100% (1)
- Distillation Column Lab ReportDocument14 pagesDistillation Column Lab ReportWahida Shukori67% (3)
- Continuous Stirrer Tank Reactors in Series.Document54 pagesContinuous Stirrer Tank Reactors in Series.Farrukh Shahzad60% (5)
- Abstract For CSTR Lab ReportDocument4 pagesAbstract For CSTR Lab ReportNabilah SyaheeraNo ratings yet
- CSTRDocument12 pagesCSTRsamueloNo ratings yet
- Tubular ReactorDocument20 pagesTubular ReactorMuhamad Hafifi AjwadNo ratings yet
- LabDocument16 pagesLabMuhamad Hafifi AjwadNo ratings yet
- S7 11012021 Acid Base Titrations WS With ANSWERSDocument7 pagesS7 11012021 Acid Base Titrations WS With ANSWERSFatima Ahmed-VeriterNo ratings yet
- Lube Oil Flushing Procedure - Rev 0Document22 pagesLube Oil Flushing Procedure - Rev 0Yusuf67% (3)
- CSTR in SeriesDocument16 pagesCSTR in SeriesAhmadAzriMohamad50% (2)
- Experiment CSTR 40LDocument18 pagesExperiment CSTR 40LSaber Minato Azrul100% (2)
- Continuous Stirred Tank Reactor (CSTR) in SeriesDocument15 pagesContinuous Stirred Tank Reactor (CSTR) in SeriesHaizul Radzi33% (3)
- CSTRDocument15 pagesCSTRbilisfreak100% (3)
- Stirred Tank in Series ReportDocument20 pagesStirred Tank in Series ReportEmonbeifo EfosasereNo ratings yet
- 5 - (CSTR Bp143)Document12 pages5 - (CSTR Bp143)Aisyah Addia AzizanNo ratings yet
- CSTR SeriesDocument14 pagesCSTR SeriesElina Nes100% (1)
- Tubular Flow Reactor ReportDocument19 pagesTubular Flow Reactor ReportN Afiqah Razak100% (1)
- CSTR in SeriesDocument12 pagesCSTR in Seriesmnizamarzuki0% (2)
- Stirred Tank ReactorDocument32 pagesStirred Tank ReactorChristopher Emeka Ominyi100% (1)
- Lab RPRT (CSTR)Document21 pagesLab RPRT (CSTR)Black White80% (5)
- CHE506 - Lab Report On Continuous Stirre PDFDocument29 pagesCHE506 - Lab Report On Continuous Stirre PDFMuhammad AimanNo ratings yet
- Results and Discussion of CSTR in SeriesDocument3 pagesResults and Discussion of CSTR in SeriesleenzalalNo ratings yet
- Chemical Reaction Engineering 40 L CSTR SaponificationDocument21 pagesChemical Reaction Engineering 40 L CSTR SaponificationMohamad Turmizi Jaafar67% (3)
- CSTRDocument19 pagesCSTRAmir Al-AimanNo ratings yet
- CSTR ReportDocument21 pagesCSTR ReportJonathon Douglas100% (1)
- CSTRDocument25 pagesCSTRAinul Mardhiah Abdul Rahim100% (1)
- CHE504 - Lab Report On Distillation ColuDocument27 pagesCHE504 - Lab Report On Distillation ColuMuhammad Irfan MalikNo ratings yet
- Lab ManualDocument24 pagesLab ManualAasia FarrukhNo ratings yet
- C4 Lab ReportDocument11 pagesC4 Lab ReportchaitanyaNo ratings yet
- Che244 Exp 1Document6 pagesChe244 Exp 1NABILA AFIEQAH NASRUDINNo ratings yet
- Batch ReactorDocument4 pagesBatch ReactorFoo Xiao BingNo ratings yet
- CEV452 Lab 2 Distillation ColumnDocument22 pagesCEV452 Lab 2 Distillation ColumnAjlaa Rahim100% (1)
- Chemical Engineering Laboratory Ii: /DT Term Is Zero SinceDocument9 pagesChemical Engineering Laboratory Ii: /DT Term Is Zero SinceKayathre Raveendran100% (1)
- CSTR Lab ReportDocument14 pagesCSTR Lab ReportAmy Farhana33% (3)
- Lab 10-Batch ReactorDocument22 pagesLab 10-Batch Reactorniraj_bairagiNo ratings yet
- Lab Report 7Document39 pagesLab Report 7Fatinnnnnn100% (2)
- Chapter 1 - Part IDocument46 pagesChapter 1 - Part IMaisarah RazaliNo ratings yet
- CSTRDocument18 pagesCSTRbond45930% (1)
- Lab 1 - CSTRDocument22 pagesLab 1 - CSTRnur athilahNo ratings yet
- PK - FKK.PPM - Manual Makmal Che565: Chemical Engineering Laboratory IiiDocument21 pagesPK - FKK.PPM - Manual Makmal Che565: Chemical Engineering Laboratory Iiibedirtupak92% (12)
- Batch Reactor PDFDocument29 pagesBatch Reactor PDFSaranya KannanNo ratings yet
- Experiment 6 CSTRDocument9 pagesExperiment 6 CSTRRicky JayNo ratings yet
- CSTRDocument21 pagesCSTRirfan hilmanNo ratings yet
- CSTR FinalDocument36 pagesCSTR FinalMuhammad Yar KhanNo ratings yet
- Continuous Stirred Tank Reactor (CSTR)Document6 pagesContinuous Stirred Tank Reactor (CSTR)Elaine PuiNo ratings yet
- Report CSTRDocument14 pagesReport CSTRniraj_bairagiNo ratings yet
- Lab 4Document18 pagesLab 4Amir Al-AimanNo ratings yet
- Fundamentals of Chemical Reaction EngineeringFrom EverandFundamentals of Chemical Reaction EngineeringRating: 2.5 out of 5 stars2.5/5 (3)
- Lab ReportDocument12 pagesLab ReportkaimanwatsoNNo ratings yet
- TFR ExperimentDocument25 pagesTFR ExperimentSiti Norbaya100% (1)
- TubularDocument15 pagesTubularSharing CaringNo ratings yet
- Lab ReportDocument25 pagesLab ReportafiqahanuwarNo ratings yet
- Tubular Reactor bp101bDocument17 pagesTubular Reactor bp101bMuhamad Hafifi AjwadNo ratings yet
- Report PFRDocument21 pagesReport PFRMuhamad Hafifi Ajwad100% (2)
- Sigma Bond: From Wikipedia, The Free EncyclopediaDocument8 pagesSigma Bond: From Wikipedia, The Free EncyclopediaMuhamad Hafifi AjwadNo ratings yet
- GRI - CBM Gas in Placasdfe AnalysisDocument146 pagesGRI - CBM Gas in Placasdfe Analysisnimbo100% (1)
- E527-12 Standard Practice For Numbering Metals and Alloys in The Unified Numbering System (UNS) PDFDocument7 pagesE527-12 Standard Practice For Numbering Metals and Alloys in The Unified Numbering System (UNS) PDFUTTAM JAINNo ratings yet
- Chapter 3 EvapotranspirationDocument20 pagesChapter 3 EvapotranspirationnimcanNo ratings yet
- Synthesis of Modified Silane Acrylic Resins and Their Physical Properties As Weather-Resistant CoatingsDocument14 pagesSynthesis of Modified Silane Acrylic Resins and Their Physical Properties As Weather-Resistant CoatingszainNo ratings yet
- Basics of Magnetic Resonance ImagingDocument17 pagesBasics of Magnetic Resonance ImagingIrinaMariaStrugariNo ratings yet
- DPP 01 Chemical Bonding MridulPandey MergedDocument4 pagesDPP 01 Chemical Bonding MridulPandey Mergedsaurabh shaurya guptaNo ratings yet
- Lab #06Document22 pagesLab #06ldlewisNo ratings yet
- Mobilgear 600 XP 680 Msds - 638450Document11 pagesMobilgear 600 XP 680 Msds - 638450Nguyen DatNo ratings yet
- Amino Acids, Peptides, and Proteins I 2013Document36 pagesAmino Acids, Peptides, and Proteins I 2013ender arslanNo ratings yet
- Ucrete RG: Heavy Duty Polyurethane Concrete For Forming Cove Bases and Renovating WallsDocument3 pagesUcrete RG: Heavy Duty Polyurethane Concrete For Forming Cove Bases and Renovating Wallssyifa latifa zahidaNo ratings yet
- Toluene (010822004)Document1 pageToluene (010822004)Harry SitumorangNo ratings yet
- Research Report 296Document53 pagesResearch Report 296marcela walterosNo ratings yet
- Laser Cutting TechnologyDocument264 pagesLaser Cutting Technologykkangari100% (4)
- Alcohols and PhenolsDocument72 pagesAlcohols and PhenolsChandra ReddyNo ratings yet
- Venturi ScrubberDocument3 pagesVenturi ScrubberRoger FernandezNo ratings yet
- Fundamental Operations in Compunding, PPTDocument32 pagesFundamental Operations in Compunding, PPTdoctorneha6691% (22)
- Chem 1206 - Chapt 1Document5 pagesChem 1206 - Chapt 1Djaimee Joyce NimesNo ratings yet
- Sintering Mechanisms of Porcelain Stoneware Tiles: Castell6N (Spain)Document13 pagesSintering Mechanisms of Porcelain Stoneware Tiles: Castell6N (Spain)volkanNo ratings yet
- Fick'S Law of DiffusionDocument10 pagesFick'S Law of DiffusiondhruvNo ratings yet
- Chesterton 891Document2 pagesChesterton 891aiindustriyahoocoidNo ratings yet
- Worksheet 1 - Measurements in Chemistry (Questions)Document5 pagesWorksheet 1 - Measurements in Chemistry (Questions)kangalbert86No ratings yet
- Chicken EggshellsDocument27 pagesChicken EggshellsBenmar L. OrterasNo ratings yet
- Furstenberg-Hagg Et Al. 2013. Plant Defense Against Insect Herbivores.Document56 pagesFurstenberg-Hagg Et Al. 2013. Plant Defense Against Insect Herbivores.J Alberto LucasNo ratings yet
- Aiadrveng: ScuijahDocument3 pagesAiadrveng: ScuijahAtom SuwiJakNo ratings yet
- f7 9 2 f780 f781 f782Document16 pagesf7 9 2 f780 f781 f782abcdsefNo ratings yet
- Centrifugation in The Pharmaceutical IndustryDocument12 pagesCentrifugation in The Pharmaceutical Industrymamun_ruNo ratings yet