Professional Documents
Culture Documents
S. Pneumoniae Produces Local Edema That Aids in The Proliferation of Organisms and Their
S. Pneumoniae Produces Local Edema That Aids in The Proliferation of Organisms and Their
Uploaded by
Kadek Soga Prayaditya PutraOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
S. Pneumoniae Produces Local Edema That Aids in The Proliferation of Organisms and Their
S. Pneumoniae Produces Local Edema That Aids in The Proliferation of Organisms and Their
Uploaded by
Kadek Soga Prayaditya PutraCopyright:
Available Formats
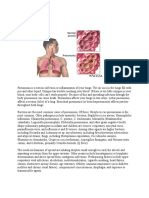
Pneumonia
PATHOGENESIS.
The lower respiratory tract is normally kept sterile by physiologic defense mechanisms,
including the mucocil iary clearance, the properties of normal secretions such as secretory
immunoglobulin A (IgA), and clearing of the airway by coughing. Immunologic defense
mechanisms of the lung that limit invasion by pathogenic organisms include macrophages
that are present in alveoli and bronchioles, secretory IgA, and other immunoglobulins.
Viral pneumonia usually results from spread of infection along the airways, accompanied by
direct injury of the respiratory epithelium, resulting in airway obstruction from swelling,
abnormal secretions, and cellular debris. The small caliber of airways in young infants makes
them particularly susceptible to severe infection. Atelectasis, interstitial edema, and
ventilation-perfusion mismatch causing significant hypoxemia often accompany airway
obstruction. Viral infection of the respiratory tract can also predispose to secondary bacterial
infection by disturbing normal host defense mechanisms, altering secretions, and modifying
the bacterial flora.
When bacterial infection is established in the lung parenchyma, the pathologic process varies
according to the invading organism. M. pneumoniae attaches to the respiratory epithelium,
inhibits ciliary action, and leads to cellular destruction and an inflammatory response in the
submucosa. As the infection progresses, sloughed cellular debris, inflammatory cells, and
mucus cause airway obstruction, with spread of infection occurring along the bronchial tree,
as it does in viral pneumonia.
S. pneumoniae produces local edema that aids in the proliferation of organisms and their
spread into adjacent portions of lung, often resulting in the characteristic focal lobar
involvement.
Group A streptococcus infection of the lower respiratory tract results in more diffuse
infection with interstitial pneumonia. The pathology includes necrosis of tracheobronchial
mucosa; formation of large amounts of exudate, edema, and local hemorrhage, with extension
into the interalveolar septa; and involvement of lymphatic vessels and the increased
likelihood of pleural involvement.
S. aureus pneumonia manifests in confluent bronchopneumonia, which is often unilateral and
characterized by the presence of extensive areas of hemorrhagic necrosis and irregular areas
of cavitation of the lung parenchyma, resulting in pneumatoceles, empyema, or, at times,
bronchopulmonary fistulas.
Recurrent pneumonia is defined as 2 or more episodes in a single yr or 3 or more episodes
ever, with radiographic clearing between occurrences.
CLINICAL MANIFESTATIONS.
Viral and bacterial pneumonias are often preceded by several days of symptoms of an upper
respiratory tract infection, typically rhinitis and cough. In viral pneu monia, fever is usually
present; temperatures are generally lower than in bacterial pneumonia. Tachypnea is the most
consistent clinical manifestation of pneumonia. Increased work of breathing accompanied by
intercostal, subcostal, and suprasternal retractions, nasal flaring, and use of accessory muscles
is common. Severe infection may be accompanied by cyanosis and respiratory fatigue,
especially in infants. Auscultation of the chest may reveal crackles and wheezing, but it is
often difficult to localize the source of these adventitious sounds in very young children with
hyperresonant chests. It is often not possible to distinguish viral pneumonia clinically from
disease caused by Mycoplasma and other bacterial pathogens.
Bacterial pneumonia in adults and older children typically begins suddenly with a shaking
chill followed by a high fever, cough, and chest pain. In older children and adolescents, a
brief upper respiratory tract illness is followed by the abrupt onset of shaking chills and high
fever accompanied by drowsiness with intermittent periods of restlessness; rapid respirations;
a dry, hacking, unproductive cough; anxiety; and, occasionally, delirium. Circumoral
cyanosis may be observed. Many children are noted to be splinting on the affected side to
minimize pleuritic pain and improve ventilation; they may lie on their side with their knees
drawn up to their chest.
Physical findings depend on the stage of pneumonia. Early in the course of illness,
diminished breath sounds, scattered crackles, and rhonchi are commonly heard over the
affected lung field. With the development of increasing consolidation or complications of
pneumonia such as effusion, empyema, or pyopneumothorax, dullness on percussion is noted
and breath sounds may be diminished. A lag in respiratory excursion often occurs on the
affected side. Abdominal distention may be prominent because of gastric dilation from
swallowed air or ileus. Abdominal pain is common in lower lobe pneumonia. The liver may
seem enlarged because of downward displacement of the diaphragm secondary to
hyperinflation of the lungs or superimposed congestive heart failure. Nuchal rigidity, in the
absence of meningitis, may also be prominent, especially with involvement of the right upper
lobe.
Symptoms described in adults with pneumococcal pneumonia may be noted in older children
but are rarely observed in infants and young children, in whom the clinical pattern is
considerably more variable. In infants, there may be a prodrome of upper respiratory tract
infection and diminished appetite, leading to the abrupt onset of fever, restlessness,
apprehension, and respiratory distress. These infants appear ill with respiratory distress
manifested by grunting; nasal flaring; retractions of the supraclavicular, intercostal, and
subcostal areas; tachypnea; tachycardia; air hunger; and often cyanosis. Results of physical
examination may be misleading, particularly in young infants, with meager findings
disproportionate to the degree of tachypnea. Some infants with bacterial pneumonia may
have associated gastrointestinal disturbances characterized by vomiting, anorexia, diarrhea,
and abdominal distention secondary to a paralytic ileus. Rapid progression of symptoms is
characteristic in the most severe cases of bacterial pneumonia
DIAGNOSIS.
The chest radiograph confirms the diagnosis of pneumonia and may indicate a complication
such as a pleural effusion or empyema. Viral pneumonia is usually characterized by
hyperinflation with bilateral interstitial infiltrates and peribronchial cuffing ( Fig. 397-1 ).
Confluent lobar consolidation is typically seen with pneumococcal pneumonia ( Fig. 397-2 ).
The radiographic appearance alone is not diagnostic and other clinical features must be
considered. Repeat chest x-rays are not required for proof of cure for patients with
uncomplicated pneumonia.
The peripheral white blood cell (WBC) count can be useful in differentiating viral from
bacterial pneumonia. In viral pneumonia, the WBC count can be normal or elevated but is
usually not higher than 20,000/mm
3
, with a lymphocyte predominance. Bacterial pneumonia
(occasionally, adenovirus pneumonia) is often associated with an elevated WBC count in the
range of 15,000-40,000/mm
3
and a predominance of granulocytes. A large pleural effusion,
lobar consolidation, and a high fever at the onset of the illness are also suggestive of a
bacterial etiology. Atypical pneumonia due to C. pneumoniae or M. pneumoniae is difficult
to distinguish from pneumococcal pneumonia by x-ray and other labs, and although
pneumococcal pneumonia is associated with a higher WBC count, erythrocyte sedimentation
rate (ESR), and Creactive protein (CRP), there is considerable overlap.
The definitive diagnosis of a viral infection rests on the isolation of a virus or detection of the
viral genome or antigen in respiratory tract secretions. Growth of respiratory viruses in tissue
culture usually requires 510 days. Reliable DNA or RNA tests for the rapid detection of
RSV, parainfluenza, influenza, and adenoviruses are available and accurate. Serologic
techniques can also be used to diagnose a recent respiratory viral infection but generally
require testing of acute and convalescent serum samples for a rise in antibodies to a specific
viral agent. This diagnostic technique is laborious, slow, and not generally clinically useful
because the infection usually resolves by the time it is confirmed serologically. Serologic
testing may be valuable as an epidemiologic tool to define the incidence and prevalence of
the various respiratory viral pathogens.
The definitive diagnosis of a bacterial infection requires isolation of an organism from the
blood, pleural fluid, or lung. Culture of sputum is of little value in the diagnosis of
pneumonia in young children. Blood cultures are positive in only 10% of children with
pneumococcal pneumonia. In M. pneumoniae infections, cold agglutinins at titers >1 : 64 are
found in the blood in 50% of patients. Cold agglutinins are nonspecific, however, because
other pathogens such as influenza viruses may also cause increases. Acute infection caused
by M. pneumoniae can be diagnosed on the basis of a positive PCR test or seroconversion in
an IgG assay. Serologic evidence such as the anti-streptolysin O (ASO) titer may be useful in
the diagnosis of group A streptococcal pneumonia
Atelektasis
Definition
Atelectasis is a collapse in part of the lungs. Normally, air passes through the airways into small sacs
of the lungs. Oxygen from the air passes through these sacs into the blood. Carbon dioxide also
passes from the blood to the sacs to leave the body. With atelectasis, these sacs are collapsed.
Oxygen and carbon dioxide cannot pass through the collapsed sacs.
A collapse over large areas of the lungs can lead to serious problems. In infants, atelectasis may be:
Congenitalpresent at birth
Acquiredcaused by an acquired condition
Causes
Atelectasis is not a disease. It is the result of a disease or abnormality in the body. It can be caused
by:
Blockage in airwaysas a result of an inhaled stool during birth, inhaled object, or a mucus plug
that keeps air from moving into sacs
Lung infectionsmay cause fluid build-up that blocks air to lung sacs
Lack of surfactant (common in premature infants)surfactant is a fluid that lines the inside of the
lungs and helps them function properly
Impaired breathingair is not pulled deep enough into the lungs to open all sacs
Damage to nerve and muscles that control breathingmay prevent coughing, deep breathing, or
yawning
*
Risk Factors
Factors that increase the chance of congenital atelectasis include:
Inhaled meconium or amniotic fluid
Prolonged or difficult labor
Birth injury to central nervous system
Children under 3 years old are more likely to develop atelectasis than older children or adults. Factors
that may increase your babys chance of atelectasis include:
Premature birth
Lung condition or infectionparticularly if coughing is impaired
Injury to chest wall
Having anesthesia
Inhaling a foreign object, such as a peanut or marble
Respiratory distress syndrome
Being on ventilatorair does not move into lungs in a normal pattern
*
Symptoms
Atelectasis may not have obvious symptoms.
Larger areas of atelectasis may lead to:
Rapid breathing
Taking shallow breaths
Agitation
Coughing
Decreased chest movement during breathing
Wheezing
Fever
Blueness of the skin
*
PATHOPHYSIOLOGY
In order to understand the pathophysiology of atelectasis, a review of the normal mechanisms that
prevent lung collapse is required. Elastin fibers within alveolar walls result in a tendency for the alveoli
to recoil inwards. This is balanced by the outward recoil of the chest wall and the interaction between
the parietal and visceral pleura. In addition, the primary mechanism that keeps the lung from
collapsing at low volumes is surfactant.
Surfactant is a complex phospholipid and protein mixture produced by type II alveolar epithelial cells.
It functions to lower surface tension, which is defined by the law of Laplace:
Pressure = 2 x (surface tension) / radius
As alveolar volume decreases with expiration, surface tension increases to the degree that, without
surfactant, the alveolus would collapse completely. This adhesive atelectasis becomes clinically
relevant in diseases that result in absent or deficient surfactant production and homeostasis, including
neonatal respiratory distress syndrome, congenital surfactant protein B deficiency, and pulmonary
alveolar proteinosis.
Obstructive atelectasis
Following obstruction of a bronchus, the circulating blood absorbs the gas in the peripheral alveoli,
leading to retraction of the lung and an airless state within a few hours. In the early stages, blood
perfuses the airless lung; this results in ventilation-perfusion mismatch and arterial hypoxemia. A
filling of the alveolar spaces with secretions and cells may occur, thereby preventing complete
collapse of the atelectatic lung. The uninvolved surrounding lung tissue distends, displacing the
surrounding structures. The heart and mediastinum shift toward the atelectatic area, the diaphragm is
elevated, and the chest wall flattens.
If the obstruction is removed, any complicating postobstructive infection subsides and the lung returns
to its normal state. If the obstruction is persistent and infection continues to be present, fibrosis
develops and the lung becomes bronchiectatic.
Nonobstructive atelectasis
The loss of contact between the visceral and parietal pleurae is the primary cause of nonobstructive
atelectasis. A pleural effusion or pneumothorax causes relaxation or passive atelectasis. Pleural
effusions affect the lower lobes more commonly than pneumothorax, which affects the upper lobes. A
large pleural-based lung mass may cause compression atelectasis by decreasing lung volumes.
Adhesive atelectasis is caused by a lack of surfactant. The surfactant has phospholipid dipalmitoyl
phosphatidylcholine, which prevents lung collapse by reducing the surface tension of the alveoli. Lack
of production or inactivation of surfactant, which may occur in ARDS, radiation pneumonitis, and blunt
trauma to the lung, cause alveolar instability and collapse.
Middle lobe syndrome (recurrent atelectasis and/or bronchiectasis involving the right middle lobe
and/or lingula) has recently been reported as the pulmonary manifestation of primary Sjgren
syndrome.
Scarring of the lung parenchyma leads to cicatrization atelectasis.
Replacement atelectasis is caused by filling of the entire lobe by a tumor such as bronchoalveolar
carcinoma.
Platelike atelectasis
Also called discoid or subsegmental atelectasis, this type is seen most commonly on chest
radiographs. Platelike atelectasis probably occurs because of obstruction of a small bronchus and is
observed in states of hypoventilation, pulmonary embolism, or lower respiratory tract infection. Small
areas of atelectasis occur because of inadequate regional ventilation and abnormalities in surfactant
formation from hypoxia, ischemia, hyperoxia, and exposure to various toxins. A mild-to-severe gas
exchange abnormality may occur because of ventilation-perfusion mismatch and intrapulmonary
shunt.
Postoperative atelectasis
Atelectasis is a common pulmonary complication in patients following thoracic and upper abdominal
procedures. General anesthesia and surgical manipulation lead to atelectasis by causing
diaphragmatic dysfunction and diminished surfactant activity. The atelectasis is typically basilar and
segmental in distribution. After induction of anesthesia, atelectasis increases from 1 to 11% of total
lung volume. End-expiratory lung volume is also found to be decreased.
In 2009 study, a recruitment maneuver plus positive end-expiratory pressure (PEEP) reduced
atelectasis to 3 4%, increased end-expiratory lung volume, and increased the PaO2/FiO2 ratio from
266 70 mm Hg to 412 99 mm Hg. It was found that the PEEP alone did not reduce the amount of
atelectasis or improve oxygenation, but a recruitment maneuver followed by PEEP reduced
atelectasis and improved oxygenation.
[4]
Medical
Lobar atelectasis is a common problem caused by a variety of mechanisms including resorption
atelectasis due to airway obstruction, passive atelectasis from hypoventilation, compressive
atelectasis from abdominal distension, and adhesive atelectasis due to increased surface tension.
Evidence-based studies on the management of lobar atelectasis are lacking. Assessment of air
bronchograms on a chest radiograph may be helpful to determine whether the airway obstruction is
proximal or distal. Chest physiotherapy, nebulized dornase alfa (DNase), and, possibly, fiberoptic
bronchoscopy might be helpful in patients with mucous plugging of the airways. In passive and
adhesive atelectasis, positive end-expiratory pressure might be a useful adjunct to treatment.
Nonpharmacologic therapies for improving cough and clearance of secretions from the airways
include chest physiotherapy, including postural drainage, chest wall percussion and vibration, and a
forced expiration technique (called huffing). Increased airway clearance as assessed by sputum
characteristics (ie, volume, weight, viscosity) and clearance of the radioaerosol from the lung show
that the long-term efficacy of these techniques compared with unassisted cough alone is unknown.
[9]
The treatment of atelectasis depends on the underlying etiology. Treatment of acute atelectasis,
including postoperative lung collapse, requires removal of the underlying cause.
For postoperative atelectasis, prevention is the best approach. Anesthetic agents associated with
postanesthesia narcosis should be avoided; narcotics should be used sparingly because they depress
the cough reflex. Early ambulation and use of incentive spirometry are important. Encourage the
patient to cough and to breathe deeply. Nebulized bronchodilators and humidity may help liquefy
secretions and promote their easy removal. In the case of lobar atelectasis, vigorous chest
physiotherapy frequently helps reexpand the collapsed lung. When these efforts are not successful
within 24 hours, flexible fiberoptic bronchoscopy should be performed.
When a mechanically obstructed bronchus is suggested but coughing or suctioning is not successful,
bronchoscopy should be performed. If bronchoscopy is successful, any underlying infection, if
present, is treated.
Prevention of further atelectasis involves (1) placing the patient in such a position that the uninvolved
side is dependent to promote increased drainage of the affected area, (2) giving vigorous chest
physiotherapy, and (3) encouraging the patient to cough and to breathe deeply.
Patients may require repeat bronchoscopy if atelectasis recurs. This is particularly true in patients with
neuromuscular disease and poor cough.
Therapy with a broad-spectrum antibiotic is started and modified appropriately if a specific pathogen
is isolated from sputum samples or bronchial secretions.
Postoperative atelectasis is treated with adequate oxygenation and reexpansion of the lung
segments. Supplemental oxygen should be titrated to achieve an arterial oxygen saturation of greater
than 90%.
Severe hypoxemia associated with severe respiratory distress or hypoxemia should lead to intubation
and mechanical support. Intubation not only provides oxygenation and ventilatory support, but also
provides access for suctioning of the airways and facilitates performing bronchoscopy, if needed. The
positive pressure and larger tidal volumes often help to reexpand collapsed lung segments.
Continuous positive airway pressure delivered via a nasal cannula or facemask may also be effective
in improving oxygenation and reexpanding the collapsed lung.
Broad-spectrum antibiotics should be prescribed if evidence of infection is present, such as fever,
night sweats, or leukocytosis, because secondary atelectasis usually becomes infected regardless of
the cause of obstruction. Obstruction of a major bronchus may cause severe hacking or coughing.
Antitussive therapy reduces the cough reflex and may produce further obstruction; thus, it should be
avoided.
Fiberoptic bronchoscopy is commonly required for diagnosis, particularly if an endobronchial lesion is
suggested. This procedure has a limited role in the management of postoperative atelectasis.
Fiberoptic bronchoscopy is not more effective than standard chest physiotherapy, deep breathing,
coughing, and suctioning of patients who are intubated. Therefore, simple and standard respiratory
therapy techniques should be administered to patients who spontaneously ventilate or patients on
mechanical ventilation. Fiberoptic bronchoscopy should be reserved for those situations in which
chest physiotherapy is contraindicated (eg, chest trauma, immobilized patient), poorly tolerated, or
unsuccessful.
Judicious use of perioperative analgesia is an essential adjunct, permitting patients to breathe deeply,
cough forcefully, and participate in chest physiotherapy maneuvers. In patients with underlying
pulmonary disease, use of epidural analgesia has been shown to be a very effective pain control
measure, thereby aiding aggressive chest physiotherapy.
N -acetylcysteine aerosols commonly are administered in an effort to promote clearance of tenacious
secretions; however, their efficacy has not been documented. In addition, N -acetylcysteine may
cause acute bronchoconstriction. Its use should be limited to direct instillation at the time of fiberoptic
bronchoscopy.
In a study of noncystic fibrosis in children who had atelectasis of infectious origin, treatment with
DNase led to rapid clinical improvement observed within 2 hours and radiologic improvement
documented within 24 hours. DNase may be an effective treatment for infectious atelectasis in
pediatric patients with noncystic fibrosis. Such data does not exist for adult patients, but DNase could
be used as a trial of therapy in adults as well.
[10]
Prophylactic maneuvers for reducing the incidence and magnitude of postoperative atelectasis in
high-risk patients should be encouraged. These techniques are deep-breathing exercises, coughing
exercises, and incentive spirometry.
For maximal benefit, prophylactic measures should be taught and instituted before surgery and used
regularly, on an hourly basis, after surgery.
Early ambulation of patients after surgery has also been found to be as effective as physical therapy.
Kato et al reported on the use of the RTX respirator for extensive atelectasis in elderly patients.
Patients were placed in the lateral decubitus position, and the RTX respirator was reported to be a
useful tool to clear retained sputum in elderly patients
You might also like
- Community Acquired Pneumonia Nelson 400Document37 pagesCommunity Acquired Pneumonia Nelson 400fatima chrystelle nuñalNo ratings yet
- Pneumonia, BronchiolitisDocument65 pagesPneumonia, BronchiolitisYemata HailuNo ratings yet
- Pediatric Community-Acquired Pneumonia: Nelson 20 EdDocument43 pagesPediatric Community-Acquired Pneumonia: Nelson 20 EdRazel Kinette AzotesNo ratings yet
- Pneumonia - Dr. SameeraDocument41 pagesPneumonia - Dr. SameeraRachanaNo ratings yet
- 17 PneumoniaDocument30 pages17 PneumoniaCabdiNo ratings yet
- Childhood PneumoniasDocument64 pagesChildhood PneumoniasestherosokoyaNo ratings yet
- LRTI and PneumoniaDocument6 pagesLRTI and Pneumoniahusenbrz4No ratings yet
- Addis Ababa University: Collage of Health Science Pediatrics and Neonatology On PneumoniaDocument21 pagesAddis Ababa University: Collage of Health Science Pediatrics and Neonatology On PneumoniaCheru DugaseNo ratings yet
- Pneumonia WW ReportDocument29 pagesPneumonia WW ReportAradhanaRamchandaniNo ratings yet
- 3 - PneumoniaDocument6 pages3 - PneumoniaAmmar AlnajjarNo ratings yet
- W1 L2 PneumoniaDocument57 pagesW1 L2 PneumoniaAnas FikriNo ratings yet
- Pcap Didactics ChanDocument61 pagesPcap Didactics ChanValerie Anne BebitaNo ratings yet
- PneumoniaDocument5 pagesPneumoniasamtaynbNo ratings yet
- PneumoniaDocument3 pagesPneumoniaJAGNo ratings yet
- Central Academy: Biology ProjectDocument12 pagesCentral Academy: Biology ProjectMastbleNo ratings yet
- Cap MRDocument4 pagesCap MRKit BarcelonaNo ratings yet
- Acute Respiratory Infections: PneumoniaDocument31 pagesAcute Respiratory Infections: PneumoniaAndy KumaraNo ratings yet
- PNEUMONIA Pathophysiology PDFDocument5 pagesPNEUMONIA Pathophysiology PDFMariaNo ratings yet
- Wae'l Hayajneh: PneumoniaDocument14 pagesWae'l Hayajneh: PneumoniaRashed ShatnawiNo ratings yet
- BronchiolitisDocument5 pagesBronchiolitisreshianeNo ratings yet
- Radiology - Chest InfectionsDocument183 pagesRadiology - Chest InfectionsRold Brio SosNo ratings yet
- File 18587Document10 pagesFile 18587Mohammed MuthanaNo ratings yet
- Lung Abscess Bronchoectasis PleurisynDocument19 pagesLung Abscess Bronchoectasis Pleurisynmarco luenaNo ratings yet
- B&lung AbscessDocument21 pagesB&lung Abscessnathan asfahaNo ratings yet
- Bronchiolitis EtiologyDocument6 pagesBronchiolitis EtiologyRonNo ratings yet
- Pediatric Community-Acquired PneumoniaDocument60 pagesPediatric Community-Acquired PneumoniaIkea Balhon100% (1)
- Bronkitis and PneumoniaDocument16 pagesBronkitis and PneumoniaRegir KurNo ratings yet
- Group 2 Community Acquired PneumoniaDocument24 pagesGroup 2 Community Acquired PneumoniaalyanadayritNo ratings yet
- 12lower Respiratory Tract InfectionDocument44 pages12lower Respiratory Tract Infectionmehdikhalid09No ratings yet
- Pneumonia Is An Inflammatory Condition of The Lung Affecting Primarily The AlveoliDocument3 pagesPneumonia Is An Inflammatory Condition of The Lung Affecting Primarily The AlveolimakkosetteNo ratings yet
- Bronchiectasis: Getnet Alemu, MD Huchs Feb 3, 2012Document38 pagesBronchiectasis: Getnet Alemu, MD Huchs Feb 3, 2012yosefNo ratings yet
- Chapter 1Document30 pagesChapter 1Ayro Business CenterNo ratings yet
- Lect 8 Pneumonia PDFDocument68 pagesLect 8 Pneumonia PDFRaghad AbdullaNo ratings yet
- Pneumatocele: BackgroundDocument8 pagesPneumatocele: BackgroundSilmi Noor RachniNo ratings yet
- Respiratory SystemDocument30 pagesRespiratory SystemHani El-asferNo ratings yet
- Pneumonia With Pleural EffusionDocument24 pagesPneumonia With Pleural EffusionMund CheleNo ratings yet
- Pneumonia in ChildrenDocument22 pagesPneumonia in ChildrenHarsha HinklesNo ratings yet
- The Wheezing Infant: Airway Problems in ChildrenDocument4 pagesThe Wheezing Infant: Airway Problems in ChildrenDenise DelaneyNo ratings yet
- InfluenzaDocument6 pagesInfluenzaVania Azalia HariyantoNo ratings yet
- PneumoniaDocument34 pagesPneumoniaAbduraman NazifNo ratings yet
- PneumoniaDocument8 pagesPneumoniaCostescu ClaudiaNo ratings yet
- Sistem Respirasi Sesak Napas: Problem Based LearningDocument61 pagesSistem Respirasi Sesak Napas: Problem Based LearningAkbar IskandarNo ratings yet
- Lung Abscess: Presented byDocument36 pagesLung Abscess: Presented byPalanki Gopal100% (1)
- Pneumonia by Kamran UOSargodhaDocument19 pagesPneumonia by Kamran UOSargodhaZeshan Haider Kazmi100% (3)
- Introduction BronchoPneumoniaDocument2 pagesIntroduction BronchoPneumoniaJanna FavilaNo ratings yet
- Diseases of Respiratory OrgansDocument72 pagesDiseases of Respiratory OrgansEdi Kerina SembiringNo ratings yet
- DifferentialsDocument7 pagesDifferentialsStewart Garneth Reston RancesNo ratings yet
- M Pneumoniae,: Community-Acquired PneumoniaDocument3 pagesM Pneumoniae,: Community-Acquired PneumoniaOlaru CatalinaNo ratings yet
- PneumoniaDocument68 pagesPneumoniaJelmalyn Basali100% (1)
- Case Study PcapDocument3 pagesCase Study PcapClaire PalaciosNo ratings yet
- BronkhiolitisDocument3 pagesBronkhiolitisKevin SandreanNo ratings yet
- Broncho PneumoniaDocument21 pagesBroncho PneumoniaRiad GagahNo ratings yet
- Cap MRDocument7 pagesCap MRZachary CohenNo ratings yet
- Chapter 3Document11 pagesChapter 3Fariz HidayatNo ratings yet
- Pneumonia CHN Case ReportDocument6 pagesPneumonia CHN Case ReportRhaine MagtotoNo ratings yet
- Argh 1Document3 pagesArgh 1acebanditsNo ratings yet
- PneumoniaDocument2 pagesPneumoniaMargaret EricksonNo ratings yet
- Upper Airway Obstruction: Ref.: Nelson Essentials of Pediatrics 7 - Edit. Nelson's Textbook of Pediatrics 20 - EditDocument5 pagesUpper Airway Obstruction: Ref.: Nelson Essentials of Pediatrics 7 - Edit. Nelson's Textbook of Pediatrics 20 - EditAli Abd AlrezaqNo ratings yet
- Medical Mnemonic Sketches : Pulmonary DiseasesFrom EverandMedical Mnemonic Sketches : Pulmonary DiseasesNo ratings yet