Professional Documents
Culture Documents
Measuring Planck's Constant: Historical Perspective and Physics Theory
Measuring Planck's Constant: Historical Perspective and Physics Theory
Uploaded by
Aruna PrasadCopyright:
Available Formats
You might also like
- Body CodeDocument305 pagesBody CodeMaynor David96% (27)
- Eisberg R & Resnick R - Quantum Physics - Atoms, Molecules, Solids, Nuclei, and Particles - Solutions Supplement Accompany (2ed Wiley)Document105 pagesEisberg R & Resnick R - Quantum Physics - Atoms, Molecules, Solids, Nuclei, and Particles - Solutions Supplement Accompany (2ed Wiley)saliya_kumara85% (34)
- Characterization of Materials: METE 310Document33 pagesCharacterization of Materials: METE 310Cezmi BacaksızNo ratings yet
- Aadrika Deokathe - Physics (Amrita Virtual Labs)Document13 pagesAadrika Deokathe - Physics (Amrita Virtual Labs)Aadrika DeokatheNo ratings yet
- Stefan Boltzmann DerivationDocument4 pagesStefan Boltzmann DerivationVlado VargasNo ratings yet
- Theory & Solved Exercise: Written/Composed By: - SHAHZAD IFTIKHAR Contact # 0313-5665666 WebsiteDocument6 pagesTheory & Solved Exercise: Written/Composed By: - SHAHZAD IFTIKHAR Contact # 0313-5665666 WebsiteFatima ObaidNo ratings yet
- Determining Plancks ConstantDocument4 pagesDetermining Plancks ConstantSimi ChNo ratings yet
- Experiment 14 The Photoelectric Effect: ObjectiveDocument4 pagesExperiment 14 The Photoelectric Effect: ObjectiveHusnain RazaNo ratings yet
- Planck ConstantDocument9 pagesPlanck ConstantMoHammed SalahNo ratings yet
- Lab 1 Plancks Constant DCEDocument5 pagesLab 1 Plancks Constant DCERaja KhanNo ratings yet
- B2 Compton Effect v3Document9 pagesB2 Compton Effect v3Mohamed RizvanNo ratings yet
- QSP Lab Manual 2024Document20 pagesQSP Lab Manual 2024Vaibhav BkNo ratings yet
- Lab 3: The Quantization of Light and Plancks Constant: 1 Photoelectic Effect ApparatusDocument8 pagesLab 3: The Quantization of Light and Plancks Constant: 1 Photoelectic Effect ApparatusshinexblazerNo ratings yet
- Manual2 Planck's ConstantDocument6 pagesManual2 Planck's ConstantRagni RanjanNo ratings yet
- FaradayDocument5 pagesFaradaysapna24No ratings yet
- 40C Lab 2 EquipotMap9Document7 pages40C Lab 2 EquipotMap9ibeupupandawayNo ratings yet
- Ece Vista: Automatic Fan ControllerDocument6 pagesEce Vista: Automatic Fan ControllerCvsrcygnusNo ratings yet
- 2 Planck's ConstantDocument6 pages2 Planck's ConstantMarie Liz AngelesNo ratings yet
- Determination of Planck's Constant: Objectives of The ExperimentDocument5 pagesDetermination of Planck's Constant: Objectives of The ExperimentkishoreputhaNo ratings yet
- Planck's Constant LABDocument3 pagesPlanck's Constant LAByaduvanshilaxman321No ratings yet
- Blackbody RadiationDocument5 pagesBlackbody RadiationLuis E Hernandez100% (1)
- Faculty ManualDocument60 pagesFaculty ManualCookiesNo ratings yet
- Efield DipoleDocument3 pagesEfield DipoleAsma TabasumNo ratings yet
- Experiment 3-7. Electric Fields and Equipotential LinesDocument10 pagesExperiment 3-7. Electric Fields and Equipotential LinesKristel PagaduanNo ratings yet
- Interpretation of ResultsDocument2 pagesInterpretation of ResultsKevin Luis Markus PinedaNo ratings yet
- Photoelectric EffectDocument6 pagesPhotoelectric Effectabc abc100% (1)
- Planck's Constant DeterminationDocument3 pagesPlanck's Constant DeterminationFarhan Ishrak AhmedNo ratings yet
- Radiatergdfgadgergeg 43trf 34frefDocument10 pagesRadiatergdfgadgergeg 43trf 34frefjack jackNo ratings yet
- Planks ConstantDocument5 pagesPlanks Constantprateekjain01100% (4)
- IntroLabVIEW StefanBoltzmann F16Document8 pagesIntroLabVIEW StefanBoltzmann F16Pfano MarandelaNo ratings yet
- Measuring Planck'c Constant Using LEDDocument4 pagesMeasuring Planck'c Constant Using LEDmarkmejia_025No ratings yet
- Student ManualDocument61 pagesStudent ManualCookiesNo ratings yet
- Franck HertzDocument6 pagesFranck HertzOlgher Santodomingo AguilarNo ratings yet
- PV Characterization Lab: PurposeDocument6 pagesPV Characterization Lab: PurposeMudit MittalNo ratings yet
- Led Planck Ex PTDocument4 pagesLed Planck Ex PTBoceNo ratings yet
- 41A: Inverse Square Law For RadiationDocument4 pages41A: Inverse Square Law For RadiationSafi Ullah KhanNo ratings yet
- Calculation of Planck Constant Using Photocell: Name: Shivam Roll No.: 20313127Document13 pagesCalculation of Planck Constant Using Photocell: Name: Shivam Roll No.: 20313127shivamNo ratings yet
- JP (V Lab - 4)Document6 pagesJP (V Lab - 4)shivampatel22098No ratings yet
- Yeditepe University Department of Electrical and Electronics Engineering Ee 232 Introduction To ElectronicsDocument5 pagesYeditepe University Department of Electrical and Electronics Engineering Ee 232 Introduction To Electronicstarafe5509No ratings yet
- RL Circuit Lab Purpose: Today You Are Going To Use The RCL Circuit Board. TheDocument2 pagesRL Circuit Lab Purpose: Today You Are Going To Use The RCL Circuit Board. Theblerb795No ratings yet
- Report 3Document8 pagesReport 3Tuan NguyenNo ratings yet
- Subject: Physics Laboratory Subject Code: Phs 383 Reg No:19U10781Document34 pagesSubject: Physics Laboratory Subject Code: Phs 383 Reg No:19U10781ShanmukhNo ratings yet
- Experiment 1: Experiment 1: Introduction To Laboratory InstrumentsDocument4 pagesExperiment 1: Experiment 1: Introduction To Laboratory InstrumentsWaseem HaiderNo ratings yet
- Determination of Planck ConsttDocument8 pagesDetermination of Planck ConsttManzar AliNo ratings yet
- LRC Circuits Resonances - George Ricarrson 2501987261Document14 pagesLRC Circuits Resonances - George Ricarrson 2501987261George RY100% (1)
- Pe EffectDocument6 pagesPe EffectmiltonNo ratings yet
- LRC CircuitsDocument10 pagesLRC CircuitsleisllyNo ratings yet
- VIT Campus: Electrical Measurement LabDocument6 pagesVIT Campus: Electrical Measurement LabAnkitNo ratings yet
- Lab #2 Ac Measurements: EE 200 - Electronic Circuits ImplementationDocument8 pagesLab #2 Ac Measurements: EE 200 - Electronic Circuits ImplementationPoyraz EmelNo ratings yet
- Introduction To Semiconductor Diode Characteristics and Parameter ExtractionDocument4 pagesIntroduction To Semiconductor Diode Characteristics and Parameter Extractionh111nsNo ratings yet
- Oscilloscope PracticalDocument5 pagesOscilloscope PracticaldebabratalogonNo ratings yet
- Hall Effect ExperimentDocument2 pagesHall Effect ExperimentscribdianvNo ratings yet
- Instituto Politécnico Nacional: Practica 3 2da Ley de KirchhoffDocument9 pagesInstituto Politécnico Nacional: Practica 3 2da Ley de KirchhoffYovanny RiveraNo ratings yet
- Annex 2 - Delivery Format - Task 2Document6 pagesAnnex 2 - Delivery Format - Task 2Oscar BahozNo ratings yet
- 4c Lab9 Photoelectric EffectDocument4 pages4c Lab9 Photoelectric Effectk03mNo ratings yet
- Circuit Analysis Lab - An Introduction To Lab EquipmentDocument3 pagesCircuit Analysis Lab - An Introduction To Lab EquipmentBrandon SimonNo ratings yet
- Electric Circuits Lab ManualDocument34 pagesElectric Circuits Lab ManualMohamad FariezNo ratings yet
- ECE 140 L C: Inear IrcuitsDocument10 pagesECE 140 L C: Inear IrcuitsPaola RamírezNo ratings yet
- Phys136 164 Semester2 Lab Quiz Study GuideDocument11 pagesPhys136 164 Semester2 Lab Quiz Study GuideengrkskNo ratings yet
- STEM: Science, Technology, Engineering and Maths Principles Teachers Pack V10From EverandSTEM: Science, Technology, Engineering and Maths Principles Teachers Pack V10No ratings yet
- Electronic Devices and Circuits: The Commonwealth and International Library: Electrical Engineering Division, Volume 1From EverandElectronic Devices and Circuits: The Commonwealth and International Library: Electrical Engineering Division, Volume 1No ratings yet
- Sable 2018 J. Phys.: Conf. Ser. 964 012007Document7 pagesSable 2018 J. Phys.: Conf. Ser. 964 012007rishNo ratings yet
- Group Theory and Its Application To Chemistry - ChemWikiDocument13 pagesGroup Theory and Its Application To Chemistry - ChemWikieasy99No ratings yet
- Quantum 'Spooky Action at A Distance' Travels at Least 10,000 Times Faster Than LightDocument2 pagesQuantum 'Spooky Action at A Distance' Travels at Least 10,000 Times Faster Than LightquantumrealmNo ratings yet
- Comp Assignment 3Document3 pagesComp Assignment 3Jorn HoekstraNo ratings yet
- 11HS - Atomic Structure - Electron Config PracticeDocument5 pages11HS - Atomic Structure - Electron Config PracticeVictoria LowmanNo ratings yet
- Revised Notes of Unit 2Document17 pagesRevised Notes of Unit 2kanishkmodi31No ratings yet
- 14 Hydrogen and Spectral LinesDocument40 pages14 Hydrogen and Spectral LinesRahajengNo ratings yet
- Electronic SpectrosDocument66 pagesElectronic SpectrosTamilan TamilNo ratings yet
- Phy 204 6 Sem4pdfDocument6 pagesPhy 204 6 Sem4pdfshreeramsharmaNo ratings yet
- Quantum Phy (U6)Document57 pagesQuantum Phy (U6)Shiu Ping WongNo ratings yet
- Cornelia GujaDocument19 pagesCornelia GujaGreta Balasoiu100% (1)
- Unit 1 - Homework Assignment 2: The Hydrogen AtomDocument2 pagesUnit 1 - Homework Assignment 2: The Hydrogen Atom周子暄No ratings yet
- All Questions PhysicsDocument7 pagesAll Questions PhysicsChirag KundaliaNo ratings yet
- 6 Electronic SpectroscopyvDocument19 pages6 Electronic SpectroscopyvKeri Gobin SamarooNo ratings yet
- 10vector Coupling of Angular MomentumDocument16 pages10vector Coupling of Angular Momentumhamlet2020No ratings yet
- Barth DissDocument129 pagesBarth DissalikaNo ratings yet
- Economical Writing McCloskey Summaries 21-31Document5 pagesEconomical Writing McCloskey Summaries 21-31Elias GarciaNo ratings yet
- Statistical MechanicsDocument77 pagesStatistical MechanicsjuanibmcomNo ratings yet
- Ls CouplingDocument30 pagesLs CouplingVatshallaNo ratings yet
- Molecular Computing PPT (Rachit N Ravneet)Document26 pagesMolecular Computing PPT (Rachit N Ravneet)Anit SachdevaNo ratings yet
- Yakeen - 2.O Atomic Structure: Xy X yDocument3 pagesYakeen - 2.O Atomic Structure: Xy X yM.Tharun KumarNo ratings yet
- Density Functional Theory Lecture NotesDocument225 pagesDensity Functional Theory Lecture NotestenpointerNo ratings yet
- Pres Kill Lecture NotesDocument321 pagesPres Kill Lecture NotesGabriel SennoNo ratings yet
- Albert Einstein - WikipediaDocument2 pagesAlbert Einstein - WikipediaGOWTHAM NEMANINo ratings yet
- Dirac Equation: Mathematical FormulationDocument14 pagesDirac Equation: Mathematical Formulationjay_dee081651No ratings yet
- Quantum Liquids PDFDocument404 pagesQuantum Liquids PDF陳琮方No ratings yet
- Physics of Nuclei and Particles ExercisesDocument7 pagesPhysics of Nuclei and Particles Exercisestsania nkdNo ratings yet
Measuring Planck's Constant: Historical Perspective and Physics Theory
Measuring Planck's Constant: Historical Perspective and Physics Theory
Uploaded by
Aruna PrasadOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Measuring Planck's Constant: Historical Perspective and Physics Theory
Measuring Planck's Constant: Historical Perspective and Physics Theory
Uploaded by
Aruna PrasadCopyright:
Available Formats
Measuring Plancks Constant
By
Martin Hackworth
Historical Perspective and Physics Theory
Max Planck (1858-1947) was born in Kiel Germany and attended schools
in Munich and Berlin. Planck was an early pioneer in the field of quantum
physics. Around 1900 Planck developed the concept of a fundamental unit of
energy, a quantum, to explain the spectral distribution of blackbody radiation.
This idea of a basic quantum of energy is fundamental to quantum mechanics of
modern physics. Planck received a Nobel Prize for his work in the early
development of quantum mechanics in 1918. Interestingly, Planck himself
remained skeptical of practical applications for quantum theory for many years.
In order to explain blackbody radiation, Planck proposed that atoms
absorb and emit radiation in discrete quantities given by
hf E n =
where:
n is an integer known as a quantum number
f is the frequency of vibration of the molecule, and
h is a constant, Planck's constant.
Planck named these discrete units of energy quanta. The smallest discrete amount
of energy radiated or absorbed by a system results from a change in state
whereby the quantum number, n, of the system changes by one.
In 1905 Albert Einstein (1879-1955) published a paper in which he used
Planck's quantization of energy principle to explain the photoelectric effect. The
photoelectric effect involves the emission of electrons from certain materials
when exposed to light and could not be explained by classical models. For this
work Einstein received the Nobel Prize in Physics in 1921.
Niels Bohr (1885-1962) used Plancks ideas on quantization of energy as a
starting point in developing the modern theory for the hydrogen atom. Robert
Millikan made the first measurement of Plancks constant in 1916. The best
current value for Planck's constant is 6.62607554 x 10
-34
J s.
In this experiment, you will use light emitting diodes (LEDs) to measure
Planck's constant. You should be familiar with semiconductors and diodes from
Modern Physics. To review: LEDs are semiconductors that emit electromagnetic
radiation in optical and near optical frequencies when a voltage is applied to
them. LEDs emit light only when the voltage is forward biased and above a
minimum threshold value. This combination of conditions creates an electron
hole pair in a diode. Electron hole pairs are charge carriers and move when
placed in an electrical potential. Thus many electron hole pairs produce a current
when placed in an electric field. Above the threshold value the current increases
exponentially with voltage.
A quanta of energy is required to create an electron hole pair and this
energy is released when an electron and a hole recombine. In most diodes this
energy is absorbed by the semiconductor as heat, but in LEDs this quanta of
energy produces a photon of discreet energy E =hf. Because multiple states may
be excited by increasing the voltage across a diode, photons of increasing
energies will be emitted with increasing voltage. Thus the light emitted by an
LED may span a range of discrete wavelengths that decrease with increasing
voltage above the threshold voltage (shorter wavelength = higher energy). We
are interested in the maximum wavelength that is determined by the minimum
energy needed to just to create an electron hole pair. It is numerically equal to the
turn on voltage of the LED. The relation between the maximum wavelength, ,
and the turn on voltage, V0, is
0
eV
hc
hf E = = =
where:
f is the frequency of the emitted photons,
c is the velocity of light,
eis the electronic charge, and
h is Planck's constant.
The maximum wavelength of the LED can be measured to a resolution of
a few nanometers with a good spectrometer. If the turn on voltage, V0, is
measured for several diodes of different color (and different maximum
wavelength, ), a graph of V0 vs. 1/ should be linear with a slope of hc/ e. An
experimental value of Planck's constant may then be determined by using the
known values of the speed of light, c, and the charge of an electron, e, and
computing h.
Procedure
The circuit that will be used to experimentally determine Planck's constant
is illustrated below.
The A input to the 750 interface measures the voltage across the 1000
resistor in series with the LED. This voltage is directly proportional to the
current through the LED, and its value is numerically equal to the current in mA.
The B input reads the total voltage across the LED and the 1000 series resistor.
The difference between these two voltages is the voltage across the LED.
To perform the experiment, the voltage probes are connected to the first
two analog inputs of the 750 interface and the Pasco Data Studio program is used
to record the voltages as the potentiometer is turned from its minimum (fully
counterclockwise) to its maximum (fully clockwise) position. In this way,
thousands of data points may be acquired in a few seconds. After the data is
collected, it is scanned to determine the least value at which the resistor voltage
becomes nonzero. This is the turn on voltage, V0, for that particular LED. By
using different LEDs of different color (different maximum wavelength, ) and
by measuring the corresponding value of V0, a table of data of V0 versus 1/ can
be developed. A graph of V0 versus 1/ is plotted which has a slope of hc/ e, from
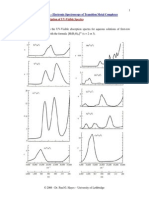
which h is determined. A typical graph of V0 versus 1/ for several LEDs is
shown below.
A
B
0.0006 0.0008 0.0010 0.0012 0.0014 0.0016 0.0018 0.0020
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
1.8
Diode V
0
(volts)
max
____________________________
IR 0.866 1000
Red 1.367 695
Yellow 1.464 660
Green 1.560 630
Slope=hc/
V
0
1/
Apparatus
PC
Pasco 750 interface
Voltage probes
Breadboard
1000 W resistor
5V DC regulated power supply
10kW potentiometer
large LEDs (blue, green, red, yellow)
infrared LED of known wavelength (if available)
connecting wires
Gratner spectrometer with grating.
1. Connect the 5V power supply to the breadboard. Use the convention black
(-) and red (+).
2. Connect the red probe of the test lead in A to its place on the breadboard
and the black probe to the ground. Connect the red probe of the test lead
in B to its place on the breadboard and the black probe to the ground.
3. Switch on the power supply and the 750 INTERFACE box.
4. Click on the Data Studio icon on the computer screen and choose the
appropriate port for the 750 INTERFACE box (COM1 or COM2).
5. Start the Data Studio program .
6. Move the pointer to the y-axis label and hold the left mouse button and
choose Port 1 on the menu that appears. Execute a similar procedure for
the x-axis label, only choose Port 2 for the x-axis.
7. Press and hold the right mouse button; a new menu should appear. While
holding the mouse button, move the pointer to the arrow beside "Display"
and then choose "Set All Max, Min" from the Display submenu. In the box
that appears, type 5.0 in the area for max, 0 for min, and then click "ok".
8. Repeat procedure 7 except choose "Collect" and "Data Rate". Move the
pointer to the circle in front of the number 500 and click once; click "ok".
9. Check the 10 kW potentiometer to see that it is turned fully
counterclockwise. To take data, click the "Start" button. Immediately after
clicking the Start icon, turn the screw in the center of the potentiometer
from fully counterclockwise to fully clockwise over the course of about 5
seconds. The turn should be smooth, and you should be in the fully
clockwise position by the time 5 seconds has elapsed. A typical graph is
shown below.
10. Turn off all lights in the room, and using the hand held spectrograph, look
at the LED making sure to line it up with the slit on the right hand side of
the spectrometer. You should see a band of light that covers a range of
wavelengths. Read the maximum wavelength and record the value in the
data table. Return the potentiometer to the fully counterclockwise
position.
11. Return to the Data Studio program and bring up the data table by clicking
and holding the right mouse button and select the "Window" option. From
the Window submenu, choose "Data Table A".
12. Scan the data table for the first place at which the numbers in columns 2
and 3 are both nonzero. Subtract the smaller number from the larger
number in that row and record this value as the turn on voltage for that
LED in the data table below.
13. Place the next LED in the circuit and repeat steps 9-12. Do this for each
additional LED you have until you have data for all of them. You will not
be able to measure the maximum wavelength for the infrared LED; its
value is 1000nm.
Turn on voltage
Diode voltage
VB - VA
Diode current
Data
Slope of turn on voltage versus 1/ graph __________ V m
Experimentally determined value for h _______ 10
-34
J s
Calculations
1. After exiting Data Studio, start the Graphical Analysis program by clicking
on its icon.
2. Enter your data points by choosing the "Input New Data" option and input
the information for which the program will prompt you. Plot 1/ on the
horizontal axis with units of 1/ m and the turn on voltage on the vertical axis
with units of V. (Remember your wavelengths are in nm, so if the
wavelength is 500nm, then 1/ l is 2.00 x 10
6
m
-1
, and should be entered as
2.00e6). Enter the graph title when prompted for it, and choose Automatic
Scaling, Start at 0 for both axes. Print a copy of the graph for each person in
the group.
3. The value for the slope will be given along the top of the graph. The slope is
the value for m. The value for Planck's constant is the slope of your graph
multiplied by e/ c where eis the electronic charge (1.6022 x 10
-19
C) and c is
the velocity of light (2.998 x 10
8
m/ s).
You might also like
- Body CodeDocument305 pagesBody CodeMaynor David96% (27)
- Eisberg R & Resnick R - Quantum Physics - Atoms, Molecules, Solids, Nuclei, and Particles - Solutions Supplement Accompany (2ed Wiley)Document105 pagesEisberg R & Resnick R - Quantum Physics - Atoms, Molecules, Solids, Nuclei, and Particles - Solutions Supplement Accompany (2ed Wiley)saliya_kumara85% (34)
- Characterization of Materials: METE 310Document33 pagesCharacterization of Materials: METE 310Cezmi BacaksızNo ratings yet
- Aadrika Deokathe - Physics (Amrita Virtual Labs)Document13 pagesAadrika Deokathe - Physics (Amrita Virtual Labs)Aadrika DeokatheNo ratings yet
- Stefan Boltzmann DerivationDocument4 pagesStefan Boltzmann DerivationVlado VargasNo ratings yet
- Theory & Solved Exercise: Written/Composed By: - SHAHZAD IFTIKHAR Contact # 0313-5665666 WebsiteDocument6 pagesTheory & Solved Exercise: Written/Composed By: - SHAHZAD IFTIKHAR Contact # 0313-5665666 WebsiteFatima ObaidNo ratings yet
- Determining Plancks ConstantDocument4 pagesDetermining Plancks ConstantSimi ChNo ratings yet
- Experiment 14 The Photoelectric Effect: ObjectiveDocument4 pagesExperiment 14 The Photoelectric Effect: ObjectiveHusnain RazaNo ratings yet
- Planck ConstantDocument9 pagesPlanck ConstantMoHammed SalahNo ratings yet
- Lab 1 Plancks Constant DCEDocument5 pagesLab 1 Plancks Constant DCERaja KhanNo ratings yet
- B2 Compton Effect v3Document9 pagesB2 Compton Effect v3Mohamed RizvanNo ratings yet
- QSP Lab Manual 2024Document20 pagesQSP Lab Manual 2024Vaibhav BkNo ratings yet
- Lab 3: The Quantization of Light and Plancks Constant: 1 Photoelectic Effect ApparatusDocument8 pagesLab 3: The Quantization of Light and Plancks Constant: 1 Photoelectic Effect ApparatusshinexblazerNo ratings yet
- Manual2 Planck's ConstantDocument6 pagesManual2 Planck's ConstantRagni RanjanNo ratings yet
- FaradayDocument5 pagesFaradaysapna24No ratings yet
- 40C Lab 2 EquipotMap9Document7 pages40C Lab 2 EquipotMap9ibeupupandawayNo ratings yet
- Ece Vista: Automatic Fan ControllerDocument6 pagesEce Vista: Automatic Fan ControllerCvsrcygnusNo ratings yet
- 2 Planck's ConstantDocument6 pages2 Planck's ConstantMarie Liz AngelesNo ratings yet
- Determination of Planck's Constant: Objectives of The ExperimentDocument5 pagesDetermination of Planck's Constant: Objectives of The ExperimentkishoreputhaNo ratings yet
- Planck's Constant LABDocument3 pagesPlanck's Constant LAByaduvanshilaxman321No ratings yet
- Blackbody RadiationDocument5 pagesBlackbody RadiationLuis E Hernandez100% (1)
- Faculty ManualDocument60 pagesFaculty ManualCookiesNo ratings yet
- Efield DipoleDocument3 pagesEfield DipoleAsma TabasumNo ratings yet
- Experiment 3-7. Electric Fields and Equipotential LinesDocument10 pagesExperiment 3-7. Electric Fields and Equipotential LinesKristel PagaduanNo ratings yet
- Interpretation of ResultsDocument2 pagesInterpretation of ResultsKevin Luis Markus PinedaNo ratings yet
- Photoelectric EffectDocument6 pagesPhotoelectric Effectabc abc100% (1)
- Planck's Constant DeterminationDocument3 pagesPlanck's Constant DeterminationFarhan Ishrak AhmedNo ratings yet
- Radiatergdfgadgergeg 43trf 34frefDocument10 pagesRadiatergdfgadgergeg 43trf 34frefjack jackNo ratings yet
- Planks ConstantDocument5 pagesPlanks Constantprateekjain01100% (4)
- IntroLabVIEW StefanBoltzmann F16Document8 pagesIntroLabVIEW StefanBoltzmann F16Pfano MarandelaNo ratings yet
- Measuring Planck'c Constant Using LEDDocument4 pagesMeasuring Planck'c Constant Using LEDmarkmejia_025No ratings yet
- Student ManualDocument61 pagesStudent ManualCookiesNo ratings yet
- Franck HertzDocument6 pagesFranck HertzOlgher Santodomingo AguilarNo ratings yet
- PV Characterization Lab: PurposeDocument6 pagesPV Characterization Lab: PurposeMudit MittalNo ratings yet
- Led Planck Ex PTDocument4 pagesLed Planck Ex PTBoceNo ratings yet
- 41A: Inverse Square Law For RadiationDocument4 pages41A: Inverse Square Law For RadiationSafi Ullah KhanNo ratings yet
- Calculation of Planck Constant Using Photocell: Name: Shivam Roll No.: 20313127Document13 pagesCalculation of Planck Constant Using Photocell: Name: Shivam Roll No.: 20313127shivamNo ratings yet
- JP (V Lab - 4)Document6 pagesJP (V Lab - 4)shivampatel22098No ratings yet
- Yeditepe University Department of Electrical and Electronics Engineering Ee 232 Introduction To ElectronicsDocument5 pagesYeditepe University Department of Electrical and Electronics Engineering Ee 232 Introduction To Electronicstarafe5509No ratings yet
- RL Circuit Lab Purpose: Today You Are Going To Use The RCL Circuit Board. TheDocument2 pagesRL Circuit Lab Purpose: Today You Are Going To Use The RCL Circuit Board. Theblerb795No ratings yet
- Report 3Document8 pagesReport 3Tuan NguyenNo ratings yet
- Subject: Physics Laboratory Subject Code: Phs 383 Reg No:19U10781Document34 pagesSubject: Physics Laboratory Subject Code: Phs 383 Reg No:19U10781ShanmukhNo ratings yet
- Experiment 1: Experiment 1: Introduction To Laboratory InstrumentsDocument4 pagesExperiment 1: Experiment 1: Introduction To Laboratory InstrumentsWaseem HaiderNo ratings yet
- Determination of Planck ConsttDocument8 pagesDetermination of Planck ConsttManzar AliNo ratings yet
- LRC Circuits Resonances - George Ricarrson 2501987261Document14 pagesLRC Circuits Resonances - George Ricarrson 2501987261George RY100% (1)
- Pe EffectDocument6 pagesPe EffectmiltonNo ratings yet
- LRC CircuitsDocument10 pagesLRC CircuitsleisllyNo ratings yet
- VIT Campus: Electrical Measurement LabDocument6 pagesVIT Campus: Electrical Measurement LabAnkitNo ratings yet
- Lab #2 Ac Measurements: EE 200 - Electronic Circuits ImplementationDocument8 pagesLab #2 Ac Measurements: EE 200 - Electronic Circuits ImplementationPoyraz EmelNo ratings yet
- Introduction To Semiconductor Diode Characteristics and Parameter ExtractionDocument4 pagesIntroduction To Semiconductor Diode Characteristics and Parameter Extractionh111nsNo ratings yet
- Oscilloscope PracticalDocument5 pagesOscilloscope PracticaldebabratalogonNo ratings yet
- Hall Effect ExperimentDocument2 pagesHall Effect ExperimentscribdianvNo ratings yet
- Instituto Politécnico Nacional: Practica 3 2da Ley de KirchhoffDocument9 pagesInstituto Politécnico Nacional: Practica 3 2da Ley de KirchhoffYovanny RiveraNo ratings yet
- Annex 2 - Delivery Format - Task 2Document6 pagesAnnex 2 - Delivery Format - Task 2Oscar BahozNo ratings yet
- 4c Lab9 Photoelectric EffectDocument4 pages4c Lab9 Photoelectric Effectk03mNo ratings yet
- Circuit Analysis Lab - An Introduction To Lab EquipmentDocument3 pagesCircuit Analysis Lab - An Introduction To Lab EquipmentBrandon SimonNo ratings yet
- Electric Circuits Lab ManualDocument34 pagesElectric Circuits Lab ManualMohamad FariezNo ratings yet
- ECE 140 L C: Inear IrcuitsDocument10 pagesECE 140 L C: Inear IrcuitsPaola RamírezNo ratings yet
- Phys136 164 Semester2 Lab Quiz Study GuideDocument11 pagesPhys136 164 Semester2 Lab Quiz Study GuideengrkskNo ratings yet
- STEM: Science, Technology, Engineering and Maths Principles Teachers Pack V10From EverandSTEM: Science, Technology, Engineering and Maths Principles Teachers Pack V10No ratings yet
- Electronic Devices and Circuits: The Commonwealth and International Library: Electrical Engineering Division, Volume 1From EverandElectronic Devices and Circuits: The Commonwealth and International Library: Electrical Engineering Division, Volume 1No ratings yet
- Sable 2018 J. Phys.: Conf. Ser. 964 012007Document7 pagesSable 2018 J. Phys.: Conf. Ser. 964 012007rishNo ratings yet
- Group Theory and Its Application To Chemistry - ChemWikiDocument13 pagesGroup Theory and Its Application To Chemistry - ChemWikieasy99No ratings yet
- Quantum 'Spooky Action at A Distance' Travels at Least 10,000 Times Faster Than LightDocument2 pagesQuantum 'Spooky Action at A Distance' Travels at Least 10,000 Times Faster Than LightquantumrealmNo ratings yet
- Comp Assignment 3Document3 pagesComp Assignment 3Jorn HoekstraNo ratings yet
- 11HS - Atomic Structure - Electron Config PracticeDocument5 pages11HS - Atomic Structure - Electron Config PracticeVictoria LowmanNo ratings yet
- Revised Notes of Unit 2Document17 pagesRevised Notes of Unit 2kanishkmodi31No ratings yet
- 14 Hydrogen and Spectral LinesDocument40 pages14 Hydrogen and Spectral LinesRahajengNo ratings yet
- Electronic SpectrosDocument66 pagesElectronic SpectrosTamilan TamilNo ratings yet
- Phy 204 6 Sem4pdfDocument6 pagesPhy 204 6 Sem4pdfshreeramsharmaNo ratings yet
- Quantum Phy (U6)Document57 pagesQuantum Phy (U6)Shiu Ping WongNo ratings yet
- Cornelia GujaDocument19 pagesCornelia GujaGreta Balasoiu100% (1)
- Unit 1 - Homework Assignment 2: The Hydrogen AtomDocument2 pagesUnit 1 - Homework Assignment 2: The Hydrogen Atom周子暄No ratings yet
- All Questions PhysicsDocument7 pagesAll Questions PhysicsChirag KundaliaNo ratings yet
- 6 Electronic SpectroscopyvDocument19 pages6 Electronic SpectroscopyvKeri Gobin SamarooNo ratings yet
- 10vector Coupling of Angular MomentumDocument16 pages10vector Coupling of Angular Momentumhamlet2020No ratings yet
- Barth DissDocument129 pagesBarth DissalikaNo ratings yet
- Economical Writing McCloskey Summaries 21-31Document5 pagesEconomical Writing McCloskey Summaries 21-31Elias GarciaNo ratings yet
- Statistical MechanicsDocument77 pagesStatistical MechanicsjuanibmcomNo ratings yet
- Ls CouplingDocument30 pagesLs CouplingVatshallaNo ratings yet
- Molecular Computing PPT (Rachit N Ravneet)Document26 pagesMolecular Computing PPT (Rachit N Ravneet)Anit SachdevaNo ratings yet
- Yakeen - 2.O Atomic Structure: Xy X yDocument3 pagesYakeen - 2.O Atomic Structure: Xy X yM.Tharun KumarNo ratings yet
- Density Functional Theory Lecture NotesDocument225 pagesDensity Functional Theory Lecture NotestenpointerNo ratings yet
- Pres Kill Lecture NotesDocument321 pagesPres Kill Lecture NotesGabriel SennoNo ratings yet
- Albert Einstein - WikipediaDocument2 pagesAlbert Einstein - WikipediaGOWTHAM NEMANINo ratings yet
- Dirac Equation: Mathematical FormulationDocument14 pagesDirac Equation: Mathematical Formulationjay_dee081651No ratings yet
- Quantum Liquids PDFDocument404 pagesQuantum Liquids PDF陳琮方No ratings yet
- Physics of Nuclei and Particles ExercisesDocument7 pagesPhysics of Nuclei and Particles Exercisestsania nkdNo ratings yet