Professional Documents
Culture Documents
Group 1: Basic Strength of These Hydroxides Increases As We Move Down The Group Li To Cs
Group 1: Basic Strength of These Hydroxides Increases As We Move Down The Group Li To Cs
Uploaded by
YashGoelCopyright:
Available Formats
You might also like
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Mildred MunatsiNo ratings yet
- Group 2 ElementsDocument9 pagesGroup 2 ElementsSumaya TraoreNo ratings yet
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Shujaat SiddiquiNo ratings yet
- Group II ElementsDocument15 pagesGroup II ElementsDoveNo ratings yet
- 2.6 NotesDocument6 pages2.6 NotesLisa DentonNo ratings yet
- Solubility of S-Block CompoundsDocument4 pagesSolubility of S-Block CompoundsNkemzi Elias NzetengenleNo ratings yet
- 10 Cie Group 2Document6 pages10 Cie Group 2Anshu MovvaNo ratings yet
- Module 3Document6 pagesModule 3Daneilla BanksNo ratings yet
- Detailed Notes Topic 4 Inorganic Chemistry and The Periodic Table Edexcel Chemistry A LevelDocument13 pagesDetailed Notes Topic 4 Inorganic Chemistry and The Periodic Table Edexcel Chemistry A LevelttjjjNo ratings yet
- CAIE Chemistry A-Level: 10: Group 2Document6 pagesCAIE Chemistry A-Level: 10: Group 2ahumanbeinginearthNo ratings yet
- The Solubility of Some Salts of Group Ii ElementsDocument2 pagesThe Solubility of Some Salts of Group Ii Elementscrybaby83% (6)
- Group 2Document3 pagesGroup 2ahumanbeinginearthNo ratings yet
- Group 2Document32 pagesGroup 2irnihafizan6812No ratings yet
- Group 2Document19 pagesGroup 2Muhammad KalimNo ratings yet
- S Block ElementsDocument4 pagesS Block ElementssubkitsNo ratings yet
- Group 2 Elements: UNIT 1: MOD 3 2.1-2.5Document17 pagesGroup 2 Elements: UNIT 1: MOD 3 2.1-2.5Ninti BraithwaiteNo ratings yet
- Group Trends: Prepared by K. Walker-DawkinsDocument36 pagesGroup Trends: Prepared by K. Walker-DawkinsZae ZayNo ratings yet
- The Periodic TableDocument4 pagesThe Periodic Tablekashvi kheraNo ratings yet
- Chemistry STPM Semester 2 Group 2Document7 pagesChemistry STPM Semester 2 Group 2kumutha83% (6)
- G2 SolubilityDocument2 pagesG2 SolubilityBryan AliNo ratings yet
- The S-Block ElementsDocument9 pagesThe S-Block ElementsKamal DeshapriyaNo ratings yet
- 02 Group 2 NotesDocument12 pages02 Group 2 Notesarthurmorgan09082No ratings yet
- Group 2the Alkaline Earth MetalsDocument24 pagesGroup 2the Alkaline Earth Metalsmadeee92No ratings yet
- Halogen Grp7Document7 pagesHalogen Grp718gmillsNo ratings yet
- Chemistry Unit 2, Inorganic Chemistry (2.11-2.15) Study GuideDocument22 pagesChemistry Unit 2, Inorganic Chemistry (2.11-2.15) Study Guidemannm26No ratings yet
- Akaline Earth Metals - Group 2 NotesDocument5 pagesAkaline Earth Metals - Group 2 NotesQweemyah IsraelNo ratings yet
- Group 2 and 7Document13 pagesGroup 2 and 7Nevan HuNo ratings yet
- Group 2 Notes (Sem 2)Document7 pagesGroup 2 Notes (Sem 2)Geethanjali SivakumarNo ratings yet
- Group II pOWERPOINTDocument11 pagesGroup II pOWERPOINTJays WorldNo ratings yet
- Chapter 10 Group 2Document8 pagesChapter 10 Group 2Vjayan DharmaNo ratings yet
- Chapter 11 - Group IIDocument7 pagesChapter 11 - Group IINabindra RuwaliNo ratings yet
- 2.7 The Periodic Table - Groups 2 and 7Document84 pages2.7 The Periodic Table - Groups 2 and 7Listiyaning TiasNo ratings yet
- S Block ADocument5 pagesS Block AMr BurgerNo ratings yet
- Form 2 7 Alkali MetalsDocument24 pagesForm 2 7 Alkali MetalsHarshil PatelNo ratings yet
- S BlockDocument8 pagesS BlockLightNo ratings yet
- Lecture Notes 4Document16 pagesLecture Notes 4Deandra WhitelyNo ratings yet
- Group I & IIDocument3 pagesGroup I & IINoor Ul AinNo ratings yet
- 4.04.. The Patterns Within Group 1 .Document5 pages4.04.. The Patterns Within Group 1 .Abrar JaheenNo ratings yet
- 3 1 2 Group 2Document2 pages3 1 2 Group 2Garret GordonNo ratings yet
- Group 2Document31 pagesGroup 2Shima SenseiiNo ratings yet
- 3 1 2 Group 2Document2 pages3 1 2 Group 2DecklinNo ratings yet
- Chapter 11 Group IIDocument7 pagesChapter 11 Group IICharlobabooramNo ratings yet
- S BlockDocument7 pagesS BlockpushpNo ratings yet
- S-Block Elements 2Document28 pagesS-Block Elements 2Aman SharmaNo ratings yet
- ReactionsDocument18 pagesReactionsangelinatianranNo ratings yet
- Group 2Document15 pagesGroup 2Behzod ShoraimovNo ratings yet
- Lesson 1Document19 pagesLesson 1saidbiala414No ratings yet
- C13 Properties of Metals PC SlidesDocument39 pagesC13 Properties of Metals PC SlidesBasil ChinNo ratings yet
- CHEMISTRY - Group 2Document3 pagesCHEMISTRY - Group 2annabelbithellNo ratings yet
- Group 1 ElementsDocument5 pagesGroup 1 ElementsLeong Kit WaiNo ratings yet
- Group 2 MetalsDocument19 pagesGroup 2 MetalsSelena JayyNo ratings yet
- IGCSE Chemistry - Groups 1, 7 and 0Document11 pagesIGCSE Chemistry - Groups 1, 7 and 0ChemistryKlipz100% (4)
- S - BlockDocument8 pagesS - BlockKartik ChoudharyNo ratings yet
- Periodic Table HintsDocument3 pagesPeriodic Table HintsDeepa KarthikNo ratings yet
- Thermal DecompositionDocument4 pagesThermal DecompositionDiddled_skittlesNo ratings yet
- Group 2 Part 2 EdexcelDocument2 pagesGroup 2 Part 2 EdexcelKevin The Chemistry TutorNo ratings yet
- 4.4 Elements in Group 1Document14 pages4.4 Elements in Group 1Matteau LeeNo ratings yet
- Periodic TableDocument2 pagesPeriodic TablepinkiepieNo ratings yet
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)
- The Elements of Geology; Adapted to the Use of Schools and CollegesFrom EverandThe Elements of Geology; Adapted to the Use of Schools and CollegesNo ratings yet
Group 1: Basic Strength of These Hydroxides Increases As We Move Down The Group Li To Cs
Group 1: Basic Strength of These Hydroxides Increases As We Move Down The Group Li To Cs
Uploaded by
YashGoelOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Group 1: Basic Strength of These Hydroxides Increases As We Move Down The Group Li To Cs
Group 1: Basic Strength of These Hydroxides Increases As We Move Down The Group Li To Cs
Uploaded by
YashGoelCopyright:
Available Formats
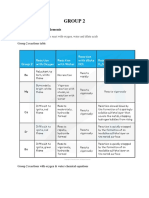
Group 1
melting points and boiling points fall as you go down the Group.
he metallic bond is weakened enough for the atoms to move around, and is
then broken completely when you boil the metal.
The fall in melting and boiling points reflects the fall in the strength of the
metallic bond.
The density tends to increase as you go down the Group (apart from the
fluctuation at potassium).
go down the Group, the carbonates become more thermally stable.(similarly
hyderogen carbonates and nitrates)
Group 1 compounds will need to be heated more strongly than those in Group
2 because the Group 1 ions are less polarising.
Group 1 compounds tend to be more soluble than the corresponding ones in
Group 2
Solubility of the carbonates increases as you go down Group 1.
Solubility of the hydroxides increases as you go down Group 1.
basic strength of these hydroxides increases as we move down the group Li to Cs.
Group 2
You will see that (apart from where the smooth trend is broken by
magnesium) the melting point falls as you go down the Group.
The hydroxides aren't very soluble, but they get more soluble as you go down
the Group.
The sulphates become less soluble as you go down the Group
The carbonates tend to become less soluble as you go down the Group.
Group 2 carbonates are virtually insoluble in water.
The carbonates become more stable to heat as you go down the Group.
The nitrates also become more stable to heat as you go down the
Group.
Solubility of the hydroxides
The hydroxides become more soluble as you go down
Group II
Solubility of sulfates
The sulfates become less soluble as you go down
Group II
Solubility of carbonates
The carbonates become less soluble as you go down
Group II
The hydroxides are Bronsted bases. Mg(OH)
2
is insoluble in water but the solubility and the strength
basicity increases going down the group
http://www.askiitians.com/iit-jee-s-and-p-block-elements/difference-between-alkaline-earth-metals-
and-alkali-metals/.
http://www.scribd.com/doc/124695438/Chemistry-Salt-Analysis-Cheat-Sheets
water of crys?
You might also like
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Mildred MunatsiNo ratings yet
- Group 2 ElementsDocument9 pagesGroup 2 ElementsSumaya TraoreNo ratings yet
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Shujaat SiddiquiNo ratings yet
- Group II ElementsDocument15 pagesGroup II ElementsDoveNo ratings yet
- 2.6 NotesDocument6 pages2.6 NotesLisa DentonNo ratings yet
- Solubility of S-Block CompoundsDocument4 pagesSolubility of S-Block CompoundsNkemzi Elias NzetengenleNo ratings yet
- 10 Cie Group 2Document6 pages10 Cie Group 2Anshu MovvaNo ratings yet
- Module 3Document6 pagesModule 3Daneilla BanksNo ratings yet
- Detailed Notes Topic 4 Inorganic Chemistry and The Periodic Table Edexcel Chemistry A LevelDocument13 pagesDetailed Notes Topic 4 Inorganic Chemistry and The Periodic Table Edexcel Chemistry A LevelttjjjNo ratings yet
- CAIE Chemistry A-Level: 10: Group 2Document6 pagesCAIE Chemistry A-Level: 10: Group 2ahumanbeinginearthNo ratings yet
- The Solubility of Some Salts of Group Ii ElementsDocument2 pagesThe Solubility of Some Salts of Group Ii Elementscrybaby83% (6)
- Group 2Document3 pagesGroup 2ahumanbeinginearthNo ratings yet
- Group 2Document32 pagesGroup 2irnihafizan6812No ratings yet
- Group 2Document19 pagesGroup 2Muhammad KalimNo ratings yet
- S Block ElementsDocument4 pagesS Block ElementssubkitsNo ratings yet
- Group 2 Elements: UNIT 1: MOD 3 2.1-2.5Document17 pagesGroup 2 Elements: UNIT 1: MOD 3 2.1-2.5Ninti BraithwaiteNo ratings yet
- Group Trends: Prepared by K. Walker-DawkinsDocument36 pagesGroup Trends: Prepared by K. Walker-DawkinsZae ZayNo ratings yet
- The Periodic TableDocument4 pagesThe Periodic Tablekashvi kheraNo ratings yet
- Chemistry STPM Semester 2 Group 2Document7 pagesChemistry STPM Semester 2 Group 2kumutha83% (6)
- G2 SolubilityDocument2 pagesG2 SolubilityBryan AliNo ratings yet
- The S-Block ElementsDocument9 pagesThe S-Block ElementsKamal DeshapriyaNo ratings yet
- 02 Group 2 NotesDocument12 pages02 Group 2 Notesarthurmorgan09082No ratings yet
- Group 2the Alkaline Earth MetalsDocument24 pagesGroup 2the Alkaline Earth Metalsmadeee92No ratings yet
- Halogen Grp7Document7 pagesHalogen Grp718gmillsNo ratings yet
- Chemistry Unit 2, Inorganic Chemistry (2.11-2.15) Study GuideDocument22 pagesChemistry Unit 2, Inorganic Chemistry (2.11-2.15) Study Guidemannm26No ratings yet
- Akaline Earth Metals - Group 2 NotesDocument5 pagesAkaline Earth Metals - Group 2 NotesQweemyah IsraelNo ratings yet
- Group 2 and 7Document13 pagesGroup 2 and 7Nevan HuNo ratings yet
- Group 2 Notes (Sem 2)Document7 pagesGroup 2 Notes (Sem 2)Geethanjali SivakumarNo ratings yet
- Group II pOWERPOINTDocument11 pagesGroup II pOWERPOINTJays WorldNo ratings yet
- Chapter 10 Group 2Document8 pagesChapter 10 Group 2Vjayan DharmaNo ratings yet
- Chapter 11 - Group IIDocument7 pagesChapter 11 - Group IINabindra RuwaliNo ratings yet
- 2.7 The Periodic Table - Groups 2 and 7Document84 pages2.7 The Periodic Table - Groups 2 and 7Listiyaning TiasNo ratings yet
- S Block ADocument5 pagesS Block AMr BurgerNo ratings yet
- Form 2 7 Alkali MetalsDocument24 pagesForm 2 7 Alkali MetalsHarshil PatelNo ratings yet
- S BlockDocument8 pagesS BlockLightNo ratings yet
- Lecture Notes 4Document16 pagesLecture Notes 4Deandra WhitelyNo ratings yet
- Group I & IIDocument3 pagesGroup I & IINoor Ul AinNo ratings yet
- 4.04.. The Patterns Within Group 1 .Document5 pages4.04.. The Patterns Within Group 1 .Abrar JaheenNo ratings yet
- 3 1 2 Group 2Document2 pages3 1 2 Group 2Garret GordonNo ratings yet
- Group 2Document31 pagesGroup 2Shima SenseiiNo ratings yet
- 3 1 2 Group 2Document2 pages3 1 2 Group 2DecklinNo ratings yet
- Chapter 11 Group IIDocument7 pagesChapter 11 Group IICharlobabooramNo ratings yet
- S BlockDocument7 pagesS BlockpushpNo ratings yet
- S-Block Elements 2Document28 pagesS-Block Elements 2Aman SharmaNo ratings yet
- ReactionsDocument18 pagesReactionsangelinatianranNo ratings yet
- Group 2Document15 pagesGroup 2Behzod ShoraimovNo ratings yet
- Lesson 1Document19 pagesLesson 1saidbiala414No ratings yet
- C13 Properties of Metals PC SlidesDocument39 pagesC13 Properties of Metals PC SlidesBasil ChinNo ratings yet
- CHEMISTRY - Group 2Document3 pagesCHEMISTRY - Group 2annabelbithellNo ratings yet
- Group 1 ElementsDocument5 pagesGroup 1 ElementsLeong Kit WaiNo ratings yet
- Group 2 MetalsDocument19 pagesGroup 2 MetalsSelena JayyNo ratings yet
- IGCSE Chemistry - Groups 1, 7 and 0Document11 pagesIGCSE Chemistry - Groups 1, 7 and 0ChemistryKlipz100% (4)
- S - BlockDocument8 pagesS - BlockKartik ChoudharyNo ratings yet
- Periodic Table HintsDocument3 pagesPeriodic Table HintsDeepa KarthikNo ratings yet
- Thermal DecompositionDocument4 pagesThermal DecompositionDiddled_skittlesNo ratings yet
- Group 2 Part 2 EdexcelDocument2 pagesGroup 2 Part 2 EdexcelKevin The Chemistry TutorNo ratings yet
- 4.4 Elements in Group 1Document14 pages4.4 Elements in Group 1Matteau LeeNo ratings yet
- Periodic TableDocument2 pagesPeriodic TablepinkiepieNo ratings yet
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)
- The Elements of Geology; Adapted to the Use of Schools and CollegesFrom EverandThe Elements of Geology; Adapted to the Use of Schools and CollegesNo ratings yet