Professional Documents
Culture Documents
Physics Prac 1 Experiment
Physics Prac 1 Experiment
Uploaded by
DirkMyburgh0 ratings0% found this document useful (0 votes)
29 views1 pageThe document summarizes an experiment to determine the density of an irregular object. The experiment involved measuring the mass of the object and determining its volume by measuring the displacement of water in a measuring jar. The density was then calculated using the mass and volume measurements. The average density calculated from five trials was 2570 kg/m3. The conclusion states that the given substance has a density of approximately 2570 kg/m3 and notes that human error could influence the results.
Original Description:
A physics practical completed
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document summarizes an experiment to determine the density of an irregular object. The experiment involved measuring the mass of the object and determining its volume by measuring the displacement of water in a measuring jar. The density was then calculated using the mass and volume measurements. The average density calculated from five trials was 2570 kg/m3. The conclusion states that the given substance has a density of approximately 2570 kg/m3 and notes that human error could influence the results.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
29 views1 pagePhysics Prac 1 Experiment
Physics Prac 1 Experiment
Uploaded by
DirkMyburghThe document summarizes an experiment to determine the density of an irregular object. The experiment involved measuring the mass of the object and determining its volume by measuring the displacement of water in a measuring jar. The density was then calculated using the mass and volume measurements. The average density calculated from five trials was 2570 kg/m3. The conclusion states that the given substance has a density of approximately 2570 kg/m3 and notes that human error could influence the results.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 1
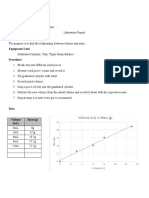
Dirk Myburgh Physics 1: Practical 1 17/02/2012
Experiment 1.2 Density and Relative Density
Aim: The aim of this experiment is to determine the density of an irregular object.
Method: A measuring jar was filled half with water, and the volume noted. A given substance
mass was measured and noted before it was dropped into the measuring jar. The
new volume was then noted. The difference between the two water levels is the
volume of the given substance. Using the mass and the volume of the substance the
density is calculated.
Results:
The density of the object is calculated through the following formula:
The mass is in kg, and
the volume is in cubic meters. The reading in ml/cm
3
is converted to m
3
. The mass of the substance
is 1.224x10
-3
kg.
No. Initial reading (a) Final reading (b) Volume (b-a) Density (kg/m
3
)
1 9 ml 9.5 ml 0.5 ml 2448
2 5.4 ml 5.9 ml 0.5 ml 2448
3 7.5 ml 7.9 ml 0.4 ml 3060
4 5.7 ml 6.2 ml 0.5 ml 2448
5 6.1 ml 6.6 ml 0.5 ml 2448
Average 2570
The density of the stone is calculated as follows:
= 2570 kg/m
3
Conclusion: The calculations have shown that the given substance has a density of 0.2402 kg/m
3
.
These densities are only approximate, since human error could influence the results.
Human errors include not working accurately, or the instruments used could have
been calibrated wrong.
You might also like
- Lab 2 Density of WaterDocument3 pagesLab 2 Density of Waterredraideratc80% (5)
- Physics Lab #1 Relative DensityDocument3 pagesPhysics Lab #1 Relative DensityAkili Armani0% (1)
- Chem 136-LAB BDocument2 pagesChem 136-LAB Bsidro12380% (5)
- Density, Statistical Analysis of Data, Graphical Data Analysis and Spreadsheet DemonstrationDocument12 pagesDensity, Statistical Analysis of Data, Graphical Data Analysis and Spreadsheet DemonstrationMiguel Ackah-Yensu75% (12)
- Written Report 4Document4 pagesWritten Report 4Raisa PailanNo ratings yet
- Enggchemlab Review QuestionsDocument3 pagesEnggchemlab Review Questionslabonetelabs21No ratings yet
- Q1 Week 3 DensityDocument7 pagesQ1 Week 3 Densityyesha arlertNo ratings yet
- Experiment 3, Mesurement of Density NewDocument2 pagesExperiment 3, Mesurement of Density NewlpborrajoNo ratings yet
- Scientific Method & Units of Measurement-2Document7 pagesScientific Method & Units of Measurement-2luigigavino7No ratings yet
- Chapter 1 Measurements: 1.7 DensityDocument21 pagesChapter 1 Measurements: 1.7 DensityUli Gi BustilloxNo ratings yet
- Chem11 Lab1 DensityDocument6 pagesChem11 Lab1 DensitynamieNo ratings yet
- V M Mass: Chemistry I and Laboratory (HS 105) Lab Experiment: Density DeterminationDocument5 pagesV M Mass: Chemistry I and Laboratory (HS 105) Lab Experiment: Density DeterminationiiohnsoiiNo ratings yet
- Name Class Period Lab # and TitleDocument2 pagesName Class Period Lab # and TitleRandy TanNo ratings yet
- L1 Density Y10Document43 pagesL1 Density Y10ellelikesheartsNo ratings yet
- DensityDocument39 pagesDensitymahmudswordofjusticeNo ratings yet
- Experiment 4Document5 pagesExperiment 4chsullivan17No ratings yet
- Name: Date: Year and Section: TeacherDocument4 pagesName: Date: Year and Section: Teacherdiana_montorioNo ratings yet
- Chem Biol Lesson 4-6Document62 pagesChem Biol Lesson 4-6siahjocsonNo ratings yet
- Dang Nguyen Exp 1 Measurements-1Document5 pagesDang Nguyen Exp 1 Measurements-1Nguyễn Hoàng ĐăngNo ratings yet
- Density and Specific GravityDocument3 pagesDensity and Specific GravityChi KoyNo ratings yet
- Liquid Physical ElementDocument4 pagesLiquid Physical ElementNurul Shha FeeqaNo ratings yet
- finalpasafinalCABO CHM171.1 LABREPORTDocument7 pagesfinalpasafinalCABO CHM171.1 LABREPORTMyra Jane CaboNo ratings yet
- Density Experiment 2 General ChemDocument8 pagesDensity Experiment 2 General ChemKudzai MashayaNo ratings yet
- Lab 1 (EARTH SCI)Document5 pagesLab 1 (EARTH SCI)Alyssa Jhoy Yacapin BracerosNo ratings yet
- AdGE General Chemistry Assessment Guide Experiment Laboratory Techniques SalvadorDocument7 pagesAdGE General Chemistry Assessment Guide Experiment Laboratory Techniques SalvadorMonis Neslie RaeNo ratings yet
- Bmmv1013 Lab 2Document18 pagesBmmv1013 Lab 2Blue BeatleNo ratings yet
- Mass Weight and DensityDocument3 pagesMass Weight and DensityChikuta ShingaliliNo ratings yet
- Physics Lab Report #1 - GUIDEDocument4 pagesPhysics Lab Report #1 - GUIDEAngel MancenidoNo ratings yet
- Chemistry Lab Report 2Document10 pagesChemistry Lab Report 2Lacey Jaye Berry80% (5)
- Cobb TestDocument7 pagesCobb TestAndrei MurillonNo ratings yet
- PET524 1b PorosityDocument8 pagesPET524 1b PorosityzsiddiquiNo ratings yet
- Density LabDocument3 pagesDensity LabshahirahusninNo ratings yet
- Determination-of-Densities) Revised Activity #4Document4 pagesDetermination-of-Densities) Revised Activity #4ZENEESHA LADJAHASANNo ratings yet
- EXPERIMENT Density of SolidDocument4 pagesEXPERIMENT Density of SolidWanMardziyyahNo ratings yet
- Density Date: Density Mass Units of Density Solids (G/CM) Liquids (G/ML)Document2 pagesDensity Date: Density Mass Units of Density Solids (G/CM) Liquids (G/ML)Dilini WijesinghNo ratings yet
- Volume, Capacity and Density - Physics & ChemistryDocument2 pagesVolume, Capacity and Density - Physics & ChemistryIzan RincónNo ratings yet
- Edf5041-Laboratory Activity 2 - Measuring Density of Unknown LiquidDocument1 pageEdf5041-Laboratory Activity 2 - Measuring Density of Unknown Liquidapi-298553740No ratings yet
- Exp 1. Density of BeveragesDocument6 pagesExp 1. Density of BeveragesIsabelReyesNo ratings yet
- Chemistry LectureDocument47 pagesChemistry LectureDuchess DianalanNo ratings yet
- Density - Chemistry - SocraticDocument3 pagesDensity - Chemistry - Socratic4685752No ratings yet
- EQUILIBRIUM OF BODIES IN LIQUIDS-StudypaediaDocument16 pagesEQUILIBRIUM OF BODIES IN LIQUIDS-StudypaediaAdegoke TofunmiNo ratings yet
- Scientific MeasurementDocument64 pagesScientific MeasurementAbishaNo ratings yet
- CHM 1103 Lab #3 - Density (2021)Document3 pagesCHM 1103 Lab #3 - Density (2021)leeNo ratings yet
- Unit 2 - Density LabDocument3 pagesUnit 2 - Density LabLaken KellyNo ratings yet
- GenChem 1 Activity 2 - DensityDocument6 pagesGenChem 1 Activity 2 - Densityrb100% (1)
- ρ, of an object is defined as the ratio of its: Goals or objectivesDocument5 pagesρ, of an object is defined as the ratio of its: Goals or objectivesMaran Kachocha AlkldaneNo ratings yet
- GR 8 Density in Regular and Irregular ObjectsDocument21 pagesGR 8 Density in Regular and Irregular Objectsmoudgalya.kamavarapuNo ratings yet
- Ch9 SolutionsDocument41 pagesCh9 Solutionsmohimran2002No ratings yet
- Chem Lab #3Document3 pagesChem Lab #3Shane Razak 9DNo ratings yet
- 1.6b DIMENSIONAL ANALYSIS 1Document25 pages1.6b DIMENSIONAL ANALYSIS 1Guile MacaNo ratings yet
- Density Determination LabDocument10 pagesDensity Determination LabJack MurphyNo ratings yet
- Lab Report 7Document3 pagesLab Report 7hbvb9jqfgpNo ratings yet
- Measurement LabDocument4 pagesMeasurement LabHenessa GumiranNo ratings yet
- 5 .DensityDocument26 pages5 .Densityrtesdfr100% (1)
- Title: Specific Gravity, Density, and Unit Weight of Tap WaterDocument3 pagesTitle: Specific Gravity, Density, and Unit Weight of Tap WaterDave Vic SolisNo ratings yet
- Lab ReportDocument14 pagesLab Reportabdulkurimaw749No ratings yet
- Unit 1 - Introduction: Those Who Do Study Physics? Units and MeasurementsDocument9 pagesUnit 1 - Introduction: Those Who Do Study Physics? Units and MeasurementsCabdicasiis Maxamuud GuuleedNo ratings yet
- c1 HandoutDocument9 pagesc1 Handoutpik empikNo ratings yet
- Distillation Example 4 and 5Document2 pagesDistillation Example 4 and 5DirkMyburghNo ratings yet
- Bhopal Incident ReportDocument4 pagesBhopal Incident ReportDirkMyburghNo ratings yet
- Control Schemes of Unit OpsDocument3 pagesControl Schemes of Unit OpsDirkMyburghNo ratings yet
- Analytical Solution For A Double Pipe Heat Exchanger With Non Adiabatic Condition at The Outer Surface 1987 International Communications in Heat and MDocument8 pagesAnalytical Solution For A Double Pipe Heat Exchanger With Non Adiabatic Condition at The Outer Surface 1987 International Communications in Heat and MDirkMyburghNo ratings yet
- Compression RatiosDocument14 pagesCompression RatiosDirkMyburghNo ratings yet
- Jean Lèonard Marie Poiseuille PPT FINALDocument12 pagesJean Lèonard Marie Poiseuille PPT FINALDirkMyburghNo ratings yet
- Extended SurfacesDocument13 pagesExtended SurfacesDirkMyburghNo ratings yet
- The World's Water Vol 8Document483 pagesThe World's Water Vol 8DirkMyburgh100% (2)
- HOUSS PresentationDocument4 pagesHOUSS PresentationDirkMyburghNo ratings yet
- Physics Prac 9Document5 pagesPhysics Prac 9DirkMyburghNo ratings yet
- Temperature Measurement Devices, An AssignmentDocument6 pagesTemperature Measurement Devices, An AssignmentDirkMyburghNo ratings yet