Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
17 viewsChemical Reaction Engineering
Chemical Reaction Engineering
Uploaded by
Pinjala AnoopThe document discusses methods for determining the rate law of a chemical reaction from experimental concentration-time data. It describes the integral method, which involves guessing the reaction order and determining if the data fits a straight line, as well as the differential method using logarithms. The differential method is preferable as it does not require guessing the order and can determine both the order and rate constant directly. Graphical determination of the slope of concentration versus time curves is also discussed.
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You might also like
- Elliott Wave Timing Beyond Ordinary Fibonacci MethodsFrom EverandElliott Wave Timing Beyond Ordinary Fibonacci MethodsRating: 4 out of 5 stars4/5 (21)
- Pharmacokinetic Pharmacodynamic Modeling & Simulation PDFDocument70 pagesPharmacokinetic Pharmacodynamic Modeling & Simulation PDFSadia SajidNo ratings yet
- Data Sheet Air Foam ChamberDocument1 pageData Sheet Air Foam ChamberPinjala AnoopNo ratings yet
- Plan 53 BDocument2 pagesPlan 53 BPinjala AnoopNo ratings yet
- 40 Questions To Test A Data Scientist On Time SeriesDocument26 pages40 Questions To Test A Data Scientist On Time SeriesRajeshree JadhavNo ratings yet
- Standard Refinery Fuel TonsDocument2 pagesStandard Refinery Fuel TonsPinjala Anoop100% (4)
- Chemical Reaction EngineeringDocument11 pagesChemical Reaction EngineeringSanat MaitiNo ratings yet
- Lecture 9 - Collection and Analysis of Rate DataDocument13 pagesLecture 9 - Collection and Analysis of Rate DataSabrina AzharNo ratings yet
- 2Ch5 PDFDocument22 pages2Ch5 PDFBikashGuptaNo ratings yet
- Collection & Analysis of Rate DataDocument22 pagesCollection & Analysis of Rate DataAshutosh DhewalNo ratings yet
- Determination of Rate Equations From The Experimental DataDocument36 pagesDetermination of Rate Equations From The Experimental DataTalew TadesseNo ratings yet
- CRE7 Kinetics Lab Data Analysis RevDocument44 pagesCRE7 Kinetics Lab Data Analysis RevDeneshVijayNo ratings yet
- H03 - Data AnalysisDocument2 pagesH03 - Data AnalysishsieglerNo ratings yet
- Collection and Analysis of Rate Data: ObjectivesDocument18 pagesCollection and Analysis of Rate Data: ObjectivesLê MinhNo ratings yet
- 5 5Document4 pages5 5RifqiMuhammadNo ratings yet
- Chapter ThreeDocument24 pagesChapter Threeyilma wendayehuNo ratings yet
- Chemical Reaction Engineering (CRE) Is TheDocument22 pagesChemical Reaction Engineering (CRE) Is TheAmal ..No ratings yet
- Lecture 3Document53 pagesLecture 3Ankit MaharshiNo ratings yet
- CHAPTER 1 (Previously Chap 5) Rev1Document24 pagesCHAPTER 1 (Previously Chap 5) Rev1HakashiMirudoNo ratings yet
- 7 - Collection and Data AnalysisDocument39 pages7 - Collection and Data AnalysisHadeel AlrazimNo ratings yet
- Lecture 18. Serial Correlation: Testing and Estimation Testing For Serial CorrelationDocument21 pagesLecture 18. Serial Correlation: Testing and Estimation Testing For Serial CorrelationMilan DjordjevicNo ratings yet
- Topic 2 - Part 1Document19 pagesTopic 2 - Part 1ainmnrhNo ratings yet
- Collection and Analysis of Rate DataDocument24 pagesCollection and Analysis of Rate DataAfs IkhlasNo ratings yet
- CBRE Module 1 Part 3Document38 pagesCBRE Module 1 Part 3Ronima RajiveNo ratings yet
- Matlab Assignment 3Document3 pagesMatlab Assignment 3prasanth_0214No ratings yet
- Chapter 14 Lecture NotesDocument59 pagesChapter 14 Lecture NotesDavis LundNo ratings yet
- Goldfarb IdnaniDocument33 pagesGoldfarb Idnanifzhang756115100% (1)
- 9650-Article Text-35491-1-10-20120131 PDFDocument8 pages9650-Article Text-35491-1-10-20120131 PDFRamesh Kumar SinghNo ratings yet
- Signals and Systems (Tutorial Assignments)Document2 pagesSignals and Systems (Tutorial Assignments)toshgangwarNo ratings yet
- Lecture # 20 - Week # 12Document21 pagesLecture # 20 - Week # 12Sufyan KhanNo ratings yet
- Analysis of Data New 1Document5 pagesAnalysis of Data New 1BigNo ratings yet
- Analysis of Data New 1Document5 pagesAnalysis of Data New 1BigNo ratings yet
- Analysis of Data New 1Document5 pagesAnalysis of Data New 1BigNo ratings yet
- NM Ln5 6 PDE Compatibility ModeDocument44 pagesNM Ln5 6 PDE Compatibility ModeAngelo Di MariaNo ratings yet
- Chem162 Kinetics ReportDocument36 pagesChem162 Kinetics Reportcharwill1234No ratings yet
- CHE 358 Numerical Methods For Engineers: Dr. Martinson Addo NarteyDocument33 pagesCHE 358 Numerical Methods For Engineers: Dr. Martinson Addo NarteySarah AkuteyNo ratings yet
- Chemical KineticsDocument102 pagesChemical KineticsKishore SurampalliNo ratings yet
- MATLAB Applications of Trading Rules and GARCH With Wavelets AnalysisDocument11 pagesMATLAB Applications of Trading Rules and GARCH With Wavelets Analysisgiovanis3202No ratings yet
- Materi 1 Matematika TerapanDocument39 pagesMateri 1 Matematika TerapanDewiNo ratings yet
- 2023 1stMidTerm Test Preparation 2Document5 pages2023 1stMidTerm Test Preparation 2alejandroalvballinesNo ratings yet
- HIR DA R IVE RS ITY IO T: SC Ho Ol of Me CH An Ica L ND Ust Ria L Ee Rin GDocument52 pagesHIR DA R IVE RS ITY IO T: SC Ho Ol of Me CH An Ica L ND Ust Ria L Ee Rin Ghaile mariamNo ratings yet
- 3.batch ReactorDocument76 pages3.batch ReactorHarika KeshavNo ratings yet
- Numerical Continuation Scheme For Tracing The Double Bounded Homotopy For Analysing Nonlinear CircuitsDocument5 pagesNumerical Continuation Scheme For Tracing The Double Bounded Homotopy For Analysing Nonlinear CircuitsCalavera BanderillaNo ratings yet
- Example - Finding The: Rate LawDocument14 pagesExample - Finding The: Rate LawhosseinNo ratings yet
- 4.collection and Analysis of Rate Data - CHAPTER 5Document37 pages4.collection and Analysis of Rate Data - CHAPTER 5Marsya FarahNo ratings yet
- Error Analysis For IPhO ContestantsDocument11 pagesError Analysis For IPhO ContestantsnurlubekNo ratings yet
- JML ArimaDocument37 pagesJML Arimapg aiNo ratings yet
- Tutorial SAM6Document4 pagesTutorial SAM6Ditta Ria AriniNo ratings yet
- Numerical Methods For Partial Differential Equations: CAAM 452 Spring 2005Document43 pagesNumerical Methods For Partial Differential Equations: CAAM 452 Spring 2005Afolabi Eniola AbiolaNo ratings yet
- Safe and Effective Determinant Evaluation: February 25, 1994Document16 pagesSafe and Effective Determinant Evaluation: February 25, 1994David ImmanuelNo ratings yet
- Assignment 6-8Document19 pagesAssignment 6-8Abhishek Satish WaghNo ratings yet
- CSTRCOM: Isothermal Reactor With Complex ReactionDocument14 pagesCSTRCOM: Isothermal Reactor With Complex ReactionBishal LamichhaneNo ratings yet
- JR Fcs Sample 2012Document51 pagesJR Fcs Sample 2012Pradipta DebNo ratings yet
- Adama Science and Technology University Chemical Engineering DepartmentDocument51 pagesAdama Science and Technology University Chemical Engineering Departmentsurafel solomonNo ratings yet
- Syll Sample JRF (CS) (CSB) 2014Document60 pagesSyll Sample JRF (CS) (CSB) 2014prabhakargoliNo ratings yet
- Chaos, Solitons & Fractals: Oliver StrebelDocument12 pagesChaos, Solitons & Fractals: Oliver StrebelhjgajjarNo ratings yet
- Lab4 Orthogonal Contrasts and Multiple ComparisonsDocument14 pagesLab4 Orthogonal Contrasts and Multiple ComparisonsjorbelocoNo ratings yet
- Algorithm: - A Package For Estimation and Spectral Decomposition of Multivariate Autoregressive ModelsDocument6 pagesAlgorithm: - A Package For Estimation and Spectral Decomposition of Multivariate Autoregressive ModelsKush Kumar SinghNo ratings yet
- Analysing Batch Reactor DataDocument3 pagesAnalysing Batch Reactor DatautpalmtbiNo ratings yet
- General Instructions: Pie Matlab Assessment For Chemical EngineersDocument6 pagesGeneral Instructions: Pie Matlab Assessment For Chemical EngineersJulia RodriguezNo ratings yet
- Document 73Document38 pagesDocument 73Pinjala AnoopNo ratings yet
- General Considerations: Design PracticesDocument21 pagesGeneral Considerations: Design PracticesPinjala Anoop100% (1)
- Data Sheet Directional ValveDocument1 pageData Sheet Directional ValvePinjala AnoopNo ratings yet
- Data Sheet Cylinder Rack & Piping ManifoldDocument1 pageData Sheet Cylinder Rack & Piping ManifoldPinjala Anoop100% (1)
- Inline Mixing JGS 210-120-1-72E: ConfidentialDocument11 pagesInline Mixing JGS 210-120-1-72E: ConfidentialPinjala AnoopNo ratings yet
- Data Sheet Discharge NozzleDocument1 pageData Sheet Discharge NozzlePinjala AnoopNo ratings yet
- Data Sheet Co2 Cylinder AssemblyDocument2 pagesData Sheet Co2 Cylinder AssemblyPinjala AnoopNo ratings yet
- Preparation of LC and LG ArrangementDocument13 pagesPreparation of LC and LG ArrangementPinjala AnoopNo ratings yet
- PSA User Meet - JaipurDocument2 pagesPSA User Meet - JaipurPinjala AnoopNo ratings yet
- Tank Mixing JGS 210-120-1-66E: ConfidentialDocument9 pagesTank Mixing JGS 210-120-1-66E: ConfidentialPinjala AnoopNo ratings yet
- OISD 166 GuidelinesDocument50 pagesOISD 166 GuidelinesPinjala AnoopNo ratings yet
- 2 Phase Flow OrificeDocument14 pages2 Phase Flow OrificePinjala AnoopNo ratings yet
- Safety Contact - Pipeline Bursted During HydrotestDocument1 pageSafety Contact - Pipeline Bursted During HydrotestPinjala AnoopNo ratings yet
- Standard Refinery Fuel TonsDocument2 pagesStandard Refinery Fuel TonsPinjala AnoopNo ratings yet
- Centrifugal Compressor Surge Control Methods PDFDocument1 pageCentrifugal Compressor Surge Control Methods PDFPinjala AnoopNo ratings yet
- Energy ConversionDocument16 pagesEnergy ConversionPinjala AnoopNo ratings yet
- OISD Check List - 1Document5 pagesOISD Check List - 1Pinjala AnoopNo ratings yet
- Name: Katakam Sandeep Reddy Mobile: 9704575353: ResumeDocument2 pagesName: Katakam Sandeep Reddy Mobile: 9704575353: ResumePinjala AnoopNo ratings yet
- 3-AAP Analysis ReportDocument11 pages3-AAP Analysis ReportPinjala AnoopNo ratings yet
- Process Variable Vs TimeDocument15 pagesProcess Variable Vs TimePinjala AnoopNo ratings yet
- Is 1448 70 1968Document9 pagesIs 1448 70 1968Pinjala AnoopNo ratings yet
- Utilization of Na-Fe-Ca Composite Promoters (Industrial Wastes) As Cascade Chain Catalysis of Coal CombustionDocument2 pagesUtilization of Na-Fe-Ca Composite Promoters (Industrial Wastes) As Cascade Chain Catalysis of Coal CombustionPinjala AnoopNo ratings yet
- CDU-I Monthly Yields 2017-18 UpdatedDocument44 pagesCDU-I Monthly Yields 2017-18 UpdatedPinjala AnoopNo ratings yet
- Membrane Separation Laboratory: Experiment SetupDocument4 pagesMembrane Separation Laboratory: Experiment SetupPinjala AnoopNo ratings yet
- Indian Institute of Technology: Experimental SetupDocument3 pagesIndian Institute of Technology: Experimental SetupPinjala AnoopNo ratings yet
- A Brief Discussion On Action of Drps in Single and Multiphase FlowsDocument2 pagesA Brief Discussion On Action of Drps in Single and Multiphase FlowsPinjala AnoopNo ratings yet
- SailuDocument18 pagesSailuPinjala AnoopNo ratings yet
Chemical Reaction Engineering
Chemical Reaction Engineering
Uploaded by
Pinjala Anoop0 ratings0% found this document useful (0 votes)
17 views12 pagesThe document discusses methods for determining the rate law of a chemical reaction from experimental concentration-time data. It describes the integral method, which involves guessing the reaction order and determining if the data fits a straight line, as well as the differential method using logarithms. The differential method is preferable as it does not require guessing the order and can determine both the order and rate constant directly. Graphical determination of the slope of concentration versus time curves is also discussed.
Original Description:
reaction engg
Original Title
L20
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses methods for determining the rate law of a chemical reaction from experimental concentration-time data. It describes the integral method, which involves guessing the reaction order and determining if the data fits a straight line, as well as the differential method using logarithms. The differential method is preferable as it does not require guessing the order and can determine both the order and rate constant directly. Graphical determination of the slope of concentration versus time curves is also discussed.
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
Download as ppt, pdf, or txt
0 ratings0% found this document useful (0 votes)
17 views12 pagesChemical Reaction Engineering
Chemical Reaction Engineering
Uploaded by
Pinjala AnoopThe document discusses methods for determining the rate law of a chemical reaction from experimental concentration-time data. It describes the integral method, which involves guessing the reaction order and determining if the data fits a straight line, as well as the differential method using logarithms. The differential method is preferable as it does not require guessing the order and can determine both the order and rate constant directly. Graphical determination of the slope of concentration versus time curves is also discussed.
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
Download as ppt, pdf, or txt
You are on page 1of 12
L20
Chemical Reaction Engineering
Till Now
Basic design equation applied to
the batch reactor, CSTR, and
PFR in terms of
Conversion, X
Concentration, C
A
Molar flow, F
A
Finding rate law:
Consider the following constant volume reaction
withdraw samples of A as a function of time.
Mole Balance:
Rate Law:
Stoichiometr
y:
Combine:
Integral Method
Assume the order, , say zero ( = 0)
Conclusion: If the data do not fall on a straight line for =0 then go
for 1
st
order reaction.
Analysis:
If the data do not fall on a straight line then what should you do?
Guess 1st Order (= 1)
Guess 2nd Order (= 2)
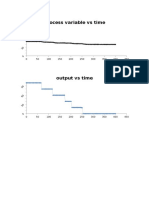
Example
Suppose your data point looks like
this picture shown in side.
Then we should stop guessing reaction orders and proceed
to either the differential method of analysis or to regression.
Differential Method
Taking the natural log of rate
equation:
alfa and k can be found from the linear
fit
(- dC
A
/ dt) what does it mean?
It is nothing but slope of the concentration vs time graph.
How can you determine the value of (- dC
A
/ dt) from CA
Vs t data?
a) Graphical
b) Polynomial
c) Finite Difference
d) Non-Linear Least Squares
Analysis
Graphical
Plot C
A
vs t
Determine the slope of this curve at suitably
selected concentration values. And this is
nothing but (- dC
A
/ dt)
Polynomial (using Polymath)
C
A
= a
o
+ a
1
t + a
2
t
2
+ a
3
t
3
+a
4
t
4
Finite Difference
Rate Law Methods
Integral Method Differentiation
No extra computation is
required
Numerical differentiation is
required
Trial and Error is required No trial and Error
Less sensitive to
Measurement error
More sensitive to
Measurement error
If order or reaction is known,
it is straight forward to find
rate constant
It is straightforward to find
both, the order and rate
constant
What Did We Learn Today?
Rate Law
Method of Integration
Method of Differentiation
Write Two things that you
understood
Write Two things that you didnt
understand
You might also like
- Elliott Wave Timing Beyond Ordinary Fibonacci MethodsFrom EverandElliott Wave Timing Beyond Ordinary Fibonacci MethodsRating: 4 out of 5 stars4/5 (21)
- Pharmacokinetic Pharmacodynamic Modeling & Simulation PDFDocument70 pagesPharmacokinetic Pharmacodynamic Modeling & Simulation PDFSadia SajidNo ratings yet
- Data Sheet Air Foam ChamberDocument1 pageData Sheet Air Foam ChamberPinjala AnoopNo ratings yet
- Plan 53 BDocument2 pagesPlan 53 BPinjala AnoopNo ratings yet
- 40 Questions To Test A Data Scientist On Time SeriesDocument26 pages40 Questions To Test A Data Scientist On Time SeriesRajeshree JadhavNo ratings yet
- Standard Refinery Fuel TonsDocument2 pagesStandard Refinery Fuel TonsPinjala Anoop100% (4)
- Chemical Reaction EngineeringDocument11 pagesChemical Reaction EngineeringSanat MaitiNo ratings yet
- Lecture 9 - Collection and Analysis of Rate DataDocument13 pagesLecture 9 - Collection and Analysis of Rate DataSabrina AzharNo ratings yet
- 2Ch5 PDFDocument22 pages2Ch5 PDFBikashGuptaNo ratings yet
- Collection & Analysis of Rate DataDocument22 pagesCollection & Analysis of Rate DataAshutosh DhewalNo ratings yet
- Determination of Rate Equations From The Experimental DataDocument36 pagesDetermination of Rate Equations From The Experimental DataTalew TadesseNo ratings yet
- CRE7 Kinetics Lab Data Analysis RevDocument44 pagesCRE7 Kinetics Lab Data Analysis RevDeneshVijayNo ratings yet
- H03 - Data AnalysisDocument2 pagesH03 - Data AnalysishsieglerNo ratings yet
- Collection and Analysis of Rate Data: ObjectivesDocument18 pagesCollection and Analysis of Rate Data: ObjectivesLê MinhNo ratings yet
- 5 5Document4 pages5 5RifqiMuhammadNo ratings yet
- Chapter ThreeDocument24 pagesChapter Threeyilma wendayehuNo ratings yet
- Chemical Reaction Engineering (CRE) Is TheDocument22 pagesChemical Reaction Engineering (CRE) Is TheAmal ..No ratings yet
- Lecture 3Document53 pagesLecture 3Ankit MaharshiNo ratings yet
- CHAPTER 1 (Previously Chap 5) Rev1Document24 pagesCHAPTER 1 (Previously Chap 5) Rev1HakashiMirudoNo ratings yet
- 7 - Collection and Data AnalysisDocument39 pages7 - Collection and Data AnalysisHadeel AlrazimNo ratings yet
- Lecture 18. Serial Correlation: Testing and Estimation Testing For Serial CorrelationDocument21 pagesLecture 18. Serial Correlation: Testing and Estimation Testing For Serial CorrelationMilan DjordjevicNo ratings yet
- Topic 2 - Part 1Document19 pagesTopic 2 - Part 1ainmnrhNo ratings yet
- Collection and Analysis of Rate DataDocument24 pagesCollection and Analysis of Rate DataAfs IkhlasNo ratings yet
- CBRE Module 1 Part 3Document38 pagesCBRE Module 1 Part 3Ronima RajiveNo ratings yet
- Matlab Assignment 3Document3 pagesMatlab Assignment 3prasanth_0214No ratings yet
- Chapter 14 Lecture NotesDocument59 pagesChapter 14 Lecture NotesDavis LundNo ratings yet
- Goldfarb IdnaniDocument33 pagesGoldfarb Idnanifzhang756115100% (1)
- 9650-Article Text-35491-1-10-20120131 PDFDocument8 pages9650-Article Text-35491-1-10-20120131 PDFRamesh Kumar SinghNo ratings yet
- Signals and Systems (Tutorial Assignments)Document2 pagesSignals and Systems (Tutorial Assignments)toshgangwarNo ratings yet
- Lecture # 20 - Week # 12Document21 pagesLecture # 20 - Week # 12Sufyan KhanNo ratings yet
- Analysis of Data New 1Document5 pagesAnalysis of Data New 1BigNo ratings yet
- Analysis of Data New 1Document5 pagesAnalysis of Data New 1BigNo ratings yet
- Analysis of Data New 1Document5 pagesAnalysis of Data New 1BigNo ratings yet
- NM Ln5 6 PDE Compatibility ModeDocument44 pagesNM Ln5 6 PDE Compatibility ModeAngelo Di MariaNo ratings yet
- Chem162 Kinetics ReportDocument36 pagesChem162 Kinetics Reportcharwill1234No ratings yet
- CHE 358 Numerical Methods For Engineers: Dr. Martinson Addo NarteyDocument33 pagesCHE 358 Numerical Methods For Engineers: Dr. Martinson Addo NarteySarah AkuteyNo ratings yet
- Chemical KineticsDocument102 pagesChemical KineticsKishore SurampalliNo ratings yet
- MATLAB Applications of Trading Rules and GARCH With Wavelets AnalysisDocument11 pagesMATLAB Applications of Trading Rules and GARCH With Wavelets Analysisgiovanis3202No ratings yet
- Materi 1 Matematika TerapanDocument39 pagesMateri 1 Matematika TerapanDewiNo ratings yet
- 2023 1stMidTerm Test Preparation 2Document5 pages2023 1stMidTerm Test Preparation 2alejandroalvballinesNo ratings yet
- HIR DA R IVE RS ITY IO T: SC Ho Ol of Me CH An Ica L ND Ust Ria L Ee Rin GDocument52 pagesHIR DA R IVE RS ITY IO T: SC Ho Ol of Me CH An Ica L ND Ust Ria L Ee Rin Ghaile mariamNo ratings yet
- 3.batch ReactorDocument76 pages3.batch ReactorHarika KeshavNo ratings yet
- Numerical Continuation Scheme For Tracing The Double Bounded Homotopy For Analysing Nonlinear CircuitsDocument5 pagesNumerical Continuation Scheme For Tracing The Double Bounded Homotopy For Analysing Nonlinear CircuitsCalavera BanderillaNo ratings yet
- Example - Finding The: Rate LawDocument14 pagesExample - Finding The: Rate LawhosseinNo ratings yet
- 4.collection and Analysis of Rate Data - CHAPTER 5Document37 pages4.collection and Analysis of Rate Data - CHAPTER 5Marsya FarahNo ratings yet
- Error Analysis For IPhO ContestantsDocument11 pagesError Analysis For IPhO ContestantsnurlubekNo ratings yet
- JML ArimaDocument37 pagesJML Arimapg aiNo ratings yet
- Tutorial SAM6Document4 pagesTutorial SAM6Ditta Ria AriniNo ratings yet
- Numerical Methods For Partial Differential Equations: CAAM 452 Spring 2005Document43 pagesNumerical Methods For Partial Differential Equations: CAAM 452 Spring 2005Afolabi Eniola AbiolaNo ratings yet
- Safe and Effective Determinant Evaluation: February 25, 1994Document16 pagesSafe and Effective Determinant Evaluation: February 25, 1994David ImmanuelNo ratings yet
- Assignment 6-8Document19 pagesAssignment 6-8Abhishek Satish WaghNo ratings yet
- CSTRCOM: Isothermal Reactor With Complex ReactionDocument14 pagesCSTRCOM: Isothermal Reactor With Complex ReactionBishal LamichhaneNo ratings yet
- JR Fcs Sample 2012Document51 pagesJR Fcs Sample 2012Pradipta DebNo ratings yet
- Adama Science and Technology University Chemical Engineering DepartmentDocument51 pagesAdama Science and Technology University Chemical Engineering Departmentsurafel solomonNo ratings yet
- Syll Sample JRF (CS) (CSB) 2014Document60 pagesSyll Sample JRF (CS) (CSB) 2014prabhakargoliNo ratings yet
- Chaos, Solitons & Fractals: Oliver StrebelDocument12 pagesChaos, Solitons & Fractals: Oliver StrebelhjgajjarNo ratings yet
- Lab4 Orthogonal Contrasts and Multiple ComparisonsDocument14 pagesLab4 Orthogonal Contrasts and Multiple ComparisonsjorbelocoNo ratings yet
- Algorithm: - A Package For Estimation and Spectral Decomposition of Multivariate Autoregressive ModelsDocument6 pagesAlgorithm: - A Package For Estimation and Spectral Decomposition of Multivariate Autoregressive ModelsKush Kumar SinghNo ratings yet
- Analysing Batch Reactor DataDocument3 pagesAnalysing Batch Reactor DatautpalmtbiNo ratings yet
- General Instructions: Pie Matlab Assessment For Chemical EngineersDocument6 pagesGeneral Instructions: Pie Matlab Assessment For Chemical EngineersJulia RodriguezNo ratings yet
- Document 73Document38 pagesDocument 73Pinjala AnoopNo ratings yet
- General Considerations: Design PracticesDocument21 pagesGeneral Considerations: Design PracticesPinjala Anoop100% (1)
- Data Sheet Directional ValveDocument1 pageData Sheet Directional ValvePinjala AnoopNo ratings yet
- Data Sheet Cylinder Rack & Piping ManifoldDocument1 pageData Sheet Cylinder Rack & Piping ManifoldPinjala Anoop100% (1)
- Inline Mixing JGS 210-120-1-72E: ConfidentialDocument11 pagesInline Mixing JGS 210-120-1-72E: ConfidentialPinjala AnoopNo ratings yet
- Data Sheet Discharge NozzleDocument1 pageData Sheet Discharge NozzlePinjala AnoopNo ratings yet
- Data Sheet Co2 Cylinder AssemblyDocument2 pagesData Sheet Co2 Cylinder AssemblyPinjala AnoopNo ratings yet
- Preparation of LC and LG ArrangementDocument13 pagesPreparation of LC and LG ArrangementPinjala AnoopNo ratings yet
- PSA User Meet - JaipurDocument2 pagesPSA User Meet - JaipurPinjala AnoopNo ratings yet
- Tank Mixing JGS 210-120-1-66E: ConfidentialDocument9 pagesTank Mixing JGS 210-120-1-66E: ConfidentialPinjala AnoopNo ratings yet
- OISD 166 GuidelinesDocument50 pagesOISD 166 GuidelinesPinjala AnoopNo ratings yet
- 2 Phase Flow OrificeDocument14 pages2 Phase Flow OrificePinjala AnoopNo ratings yet
- Safety Contact - Pipeline Bursted During HydrotestDocument1 pageSafety Contact - Pipeline Bursted During HydrotestPinjala AnoopNo ratings yet
- Standard Refinery Fuel TonsDocument2 pagesStandard Refinery Fuel TonsPinjala AnoopNo ratings yet
- Centrifugal Compressor Surge Control Methods PDFDocument1 pageCentrifugal Compressor Surge Control Methods PDFPinjala AnoopNo ratings yet
- Energy ConversionDocument16 pagesEnergy ConversionPinjala AnoopNo ratings yet
- OISD Check List - 1Document5 pagesOISD Check List - 1Pinjala AnoopNo ratings yet
- Name: Katakam Sandeep Reddy Mobile: 9704575353: ResumeDocument2 pagesName: Katakam Sandeep Reddy Mobile: 9704575353: ResumePinjala AnoopNo ratings yet
- 3-AAP Analysis ReportDocument11 pages3-AAP Analysis ReportPinjala AnoopNo ratings yet
- Process Variable Vs TimeDocument15 pagesProcess Variable Vs TimePinjala AnoopNo ratings yet
- Is 1448 70 1968Document9 pagesIs 1448 70 1968Pinjala AnoopNo ratings yet
- Utilization of Na-Fe-Ca Composite Promoters (Industrial Wastes) As Cascade Chain Catalysis of Coal CombustionDocument2 pagesUtilization of Na-Fe-Ca Composite Promoters (Industrial Wastes) As Cascade Chain Catalysis of Coal CombustionPinjala AnoopNo ratings yet
- CDU-I Monthly Yields 2017-18 UpdatedDocument44 pagesCDU-I Monthly Yields 2017-18 UpdatedPinjala AnoopNo ratings yet
- Membrane Separation Laboratory: Experiment SetupDocument4 pagesMembrane Separation Laboratory: Experiment SetupPinjala AnoopNo ratings yet
- Indian Institute of Technology: Experimental SetupDocument3 pagesIndian Institute of Technology: Experimental SetupPinjala AnoopNo ratings yet
- A Brief Discussion On Action of Drps in Single and Multiphase FlowsDocument2 pagesA Brief Discussion On Action of Drps in Single and Multiphase FlowsPinjala AnoopNo ratings yet
- SailuDocument18 pagesSailuPinjala AnoopNo ratings yet