Professional Documents
Culture Documents
Mass Balance
Mass Balance
Uploaded by
sylviealOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mass Balance
Mass Balance
Uploaded by
sylviealCopyright:
Available Formats
Paul Ashall, 2008
Module 9001
Mass Balance
Paul Ashall, 2008
The accounting of all mass in a
chemical/pharmaceutical process is referred
to as a mass (or material) balance.
Paul Ashall, 2008
Uses
day to day operation of process for
monitoring operating efficiency
Making calculations for design and
development of a process i.e. quantities
required, sizing equipment, number of items
of equipment
Paul Ashall, 2008
Simple example batch mixing
process
200 kg of a 40% w/w methanol/water solution
is mixed with 100 kg of a 70% w/w
methanol/water solution in a batch mixer
unit.
What is the final quantity and composition?
Paul Ashall, 2008
continued
Total initial mass = total final mass = 300 kg
Initial methanol mass = final methanol mass
80 + 70 = final methanol mass = 150 kg
Therefore final composition of batch is
(150/300) x 100 = 50 % by wt.
Paul Ashall, 2008
Exercise
1000 kg of 8% by wt. sodium hydroxide
(NaOH) solution is required. 20% sodium
hydroxide solution in water and pure water
are available. How much of each is
required?
Paul Ashall, 2008
Batch processes
Batch processes operate to a batch cycle and
are non-steady state. Materials are added to
a vessel in one operation and then process is
carried out and batch cycle repeated.
Integral balances are carried out on batch
processes where balances are carried out on
the initial and final states of the system.
Paul Ashall, 2008
Batch cycle
Sequence of operations/steps repeated
according to a cycle
Batch cycle time
Batch size
Paul Ashall, 2008
Simple batch reaction cycle
3 steps
Start cycle t=0 t, finish cycle
Add reactants etc reaction
Empty reactor
Next cycle
Paul Ashall, 2008
Continuous processes
These processes are continuous in nature and
operate in steady state and balances are
carried out over a fixed period of time.
Materials enter and leave process
continuously.
Paul Ashall, 2008
Law of conservation of mass
When there is no net accumulation or
depletion of mass in a system (steady state)
then:
Total mass entering system = total mass
leaving system
or total mass at start = total final mass
Paul Ashall, 2008
General mass balance equation
Input + generation output consumption =
accumulation
Notes: 1. generation and consumption terms refer only to generation of
products and consumption of reactants as a result of chemical reaction.
If there is no chemical reaction then these terms are zero.
2. Apply to a system
3. Apply to total mass and component mass
Paul Ashall, 2008
Definitions
System arbritary part or whole of a system
Steady state/non-steady state
Accumulation/depletion of mass in system
Basis for calculation of mass balance (unit
of time, batch etc)
Component or substance
Paul Ashall, 2008
Exercise
1000 kg of a 10 % by wt. sodium chloride
solution is concentrated to 50 % in a batch
evaporator. Calculate the product mass and
the mass of water evaporated from the
evaporator.
Paul Ashall, 2008
Mixing of streams
F1
F2
F3
F4
Paul Ashall, 2008
Example
Calculate E and x
Fresh feed 1000kg, 15%
by wt sodium hydrogen carbonate
Recycle stream 300 kg, 10% satd. soln.
evaporator feed E, composition x%
Paul Ashall, 2008
Flowsheets
Streams
Operations/equipment sequence
Standard symbols
Paul Ashall, 2008
Flowsheets
Process flow diagram
PID
Paul Ashall, 2008
Typical simple flowsheet
arrangement
reactor
Separation &
purification
Fresh feed
(reactants, solvents,
reagents, catalysts etc)
product
Recycle of unreacted material
Byproducts/coproducts waste
Paul Ashall, 2008
Exercise
A 1000 kg batch of a pharmaceutical powder
containing 5 % by wt water is dried in a
double cone drier. After drying 90 % of the
water has been removed. Calculate the final
batch composition and the weight of water
removed.
Paul Ashall, 2008
Exercise batch distillation
1000 kg of a 20% by wt mixture of acetone in
water is separated by multistage batch
distillation. The top product (distillate)
contains 95% by wt. acetone and the still
contains 2% acetone. Calculate the amount
of distillate.
Paul Ashall, 2008
Use of molar quantities
It is often useful to calculate a mass balance
using molar quantities of materials and to
express composition as mole fractions or
mole %.
Distillation is an example, where equilibrium
data is often expressed in mole fractions.
Paul Ashall, 2008
Molar units
A mole is the molecular weight of a substance
expressed in grams
To get the molecular weight of a substance you
need its molecular formula and you can then add
up the atomic weights of all the atoms in the
molecule
To convert from moles of a substance to grams
multiply by the molecular weight
To convert from grams to moles divide by the
molecular weight.
Mole fraction is moles divided by total moles
Mole % is mole fraction multiplied by 100
Paul Ashall, 2008
Molar units
Benzene is C
6
H
6
. The molecular weight is
(6x12) + (6x1) = 78
So 1 mole of benzene is 78 grams
1 kmol is 78 kg
Paul Ashall, 2008
Exercise batch distillation
1000 kmol of an equimolar mixture of
benzene and toluene is distilled in a
multistage batch distillation unit. 90 % of
the benzene is in the top product (distillate).
The top product has a benzene mole fraction
of 0.95. Calculate the quantities of top and
bottom products and the composition of the
bottom product.
Paul Ashall, 2008
Mass balance - crystalliser
A crystalliser contains 1000 kg of a saturated solution of potassium
chloride at 80 deg cent. It is required to crystallise 100 kg KCl from
this solution. To what temperature must the solution be cooled?
Paul Ashall, 2008
T deg cent Solubility
gKCl/100 g water
80 51.1
70 48.3
60 45.5
50 42.6
40 40
30 37
20 34
10 31
0 27.6
Paul Ashall, 2008
At 80 deg cent satd soln contains (51.1/151.1)x100
% KCl i.e. 33.8% by wt
So in 1000 kg there is 338 kg KCl & 662 kg water.
Crystallising 100 kg out of soln leaves a satd soln
containing 238 kg KCl and 662kg water i.e.
238/6.62 g KCl/100g water which is 36 g
KCl/100g. So temperature required is approx 27
deg cent from table.
Paul Ashall, 2008
Mass balance filtration/centrifuge
feed suspension
wash water/solvent
solid
waste water
filtrate
Paul Ashall, 2008
Filtration
F1
5000 kg DM water
Impurity 55 kg
Water 2600 kg
API 450 kg
Water 7300 kg
Impurity 50 kg
API 2kg
Water 300 kg
API 448 kg
Impurity 5 kg
Paul Ashall, 2008
Mass balance - drier
feed
product
water/evaporated solvent
Paul Ashall, 2008
Mass balance extraction/phase
split
A + B
S
A + B
S + B
A feed solvent; B solute; S extracting solvent
Paul Ashall, 2008
Example (single stage extraction;
immiscible solvents)
E1
feed
solvent
raffinate
extract
Paul Ashall, 2008
F = 195 kg; x
f
= 0.11 kg API/kgwater
S = 596 kg chloroform
y = 1.72x, where y is kgAPI/kg chloroform in extract and x is kg API/kg
water in raffinate.
Total balance 195 + 596 = E + R
API balance 19.5 = 175.5x
1
+ 596y
1
19.5 = 175.5x
1
+ 596.1.72x
1
x
1
= 0.0162 and y
1
= 0.029
R is 175.5 kg water + 2.84 kg API
and E is 596 kg chloroform + 17.28 kg API
Note: chloroform and water are essentially immiscible
Paul Ashall, 2008
Mass balance absorption unit
feed gas stream
feed solvent
waste solvent stream
exit gas stream
Paul Ashall, 2008
Mass balances multiple units
Overall balance
Unit balances
Component balances
Paul Ashall, 2008
Multiple units
E evaporator; C crystalliser; F filter unit
F1 fresh feed; W2 evaporated water; P3 solid product; R4 recycle
of saturated solution from filter unit
R4
E C F
F1
W2
P3
Paul Ashall, 2008
Mass balance procedures
Process description
Flowsheet
Label
Assign algebraic symbols to unknowns
(compositions, concentrations, quantities)
Select basis
Write mass balance equations (overall, total,
component, unit)
Solve equations for unknowns
Paul Ashall, 2008
Exercise
A mass balance and tracking of usage of a solvent
used in an API production process is required for a
Pollution Emission Register (PER).
Discuss and outline in general terms how you would
do this.
Ref. www.epa.ie
Paul Ashall, 2008
Definitions
Stoichiometric quantities
Limiting reactant
Excess reactant
Conversion
Yield
Selectivity
Extent of reaction
Paul Ashall, 2008
Stoichiometry
Refers to quantities of reactants and
products in a balanced chemical reaction.
aA + bB cC + dD
i.e. a moles of A react with b moles of B to
give c moles of C and d moles of D.
a,b,c,d are stoichiometric quantities
Paul Ashall, 2008
Reactor mass balances
Paul Ashall, 2008
Example aspirin synthesis
reaction
Paul Ashall, 2008
Limiting reactant/excess reactant
In practice a reactant may be used in excess
of the stoichiometric quantity for various
reasons. In this case the other reactant is
limiting i.e. it will limit the yield of
product(s)
Paul Ashall, 2008
continued
A reactant is in excess if it is present in a
quantity greater than its stoichiometric
proportion.
% excess = [(moles supplied stoichiometric
moles)/stoichiometric moles] x 100
Paul Ashall, 2008
Example aspirin synthesis
Paul Ashall, 2008
Conversion
Fractional conversion = amount reactant
consumed/amount reactant supplied
% conversion = fractional conversion x 100
Note: conversion may apply to single pass reactor
conversion or overall process conversion
Paul Ashall, 2008
Yield
Yield = (moles product/moles limiting
reactant supplied) x s.f. x 100
Where s.f. is the stoichiometric factor =
stoichiometric moles reactant required per
mole product
Paul Ashall, 2008
Example aspirin synthesis
Paul Ashall, 2008
Selectivity
Selectivity = (moles product/moles reactant
converted) x s.f. x100
OR
Selectivity = moles desired product/moles
byproduct
Paul Ashall, 2008
Extent
Extent of reaction = (moles of component leaving
reactor moles of component entering
reactor)/stoichiometric coefficient of component
Note: the stoichiometric coefficient of a component
in a chemical reaction is the no. of moles in the
balanced chemical equation ( -ve for reactants and
+ve for products)
Paul Ashall, 2008
Examples
A B
i.e. stoichiometric coefficients a = 1; b = 1
100 kmol fresh feed A; 90 % single pass
conversion in reactor; unreacted A is
separated and recycled and therefore overall
process conversion is 100%
reactor separation
F
R
P
Paul Ashall, 2008
Discussion - Synthesis of 3,3
dimethylindoline
Paul Ashall, 2008
Discussion - Aspirin synthesis
Paul Ashall, 2008
References
Elementary Principles of Chemical
Processes, R. M. Felder and R. W.
Rousseau, 3
rd
edition, John Wiley, 2000
You might also like
- SAP2000 Base Isolation PDFDocument34 pagesSAP2000 Base Isolation PDFoscavier100% (2)
- Lab Report CMT 450 Tray DryerDocument3 pagesLab Report CMT 450 Tray DryerJohanNo ratings yet
- PNG 520 - Course On Phase RelationsDocument172 pagesPNG 520 - Course On Phase RelationsDenstar Ricardo SilalahiNo ratings yet
- Test 1Document3 pagesTest 1nur hidayatiNo ratings yet
- CELCHA2 Study GuidesDocument7 pagesCELCHA2 Study GuidesEsther100% (1)
- A Theory of Dropwise CondensationDocument187 pagesA Theory of Dropwise CondensationJuvy A. BalbaronaNo ratings yet
- Exercise - Optimisation Past Year ExamDocument2 pagesExercise - Optimisation Past Year ExamAmirul AfiqNo ratings yet
- CDB 3082 Chemical Engineering Lab Iv: - Flame PropagationDocument8 pagesCDB 3082 Chemical Engineering Lab Iv: - Flame PropagationBhinitha ChandrasagaranNo ratings yet
- Flow DiagramDocument6 pagesFlow DiagramMuhammad ArshadNo ratings yet
- PowerPoint Slides of Mass Balances Section of PEME - 1030 Lectures MGDocument58 pagesPowerPoint Slides of Mass Balances Section of PEME - 1030 Lectures MGAbsi Elsaheli100% (1)
- Process Control: Designing Process and Control Systems For Dynamic PerformanceDocument29 pagesProcess Control: Designing Process and Control Systems For Dynamic PerformanceNabila Agnasia Desmara100% (1)
- 19Ch303-Chemical Process CalculationsDocument9 pages19Ch303-Chemical Process CalculationsASHADEVI UNo ratings yet
- LS-32003 Solid Liquid Extraction 80415Document32 pagesLS-32003 Solid Liquid Extraction 80415NuttyPNo ratings yet
- Double Pipe Heat ExchangerDocument3 pagesDouble Pipe Heat ExchangerSayyeda Neha FatimaNo ratings yet
- Balance On Reactive Systems LECTURE 5Document28 pagesBalance On Reactive Systems LECTURE 5OZIS AcademyNo ratings yet
- Contoh PDFDocument270 pagesContoh PDFwan100% (1)
- RECYCLEDocument34 pagesRECYCLEEmonbeifo Efosasere100% (1)
- Exp 2 Batch DistillationDocument12 pagesExp 2 Batch DistillationSabrina AzharNo ratings yet
- Material Balance Problems Involving Multiple UnitsDocument11 pagesMaterial Balance Problems Involving Multiple UnitsMay Reis BalagNo ratings yet
- Material Balances and ApplicationsDocument42 pagesMaterial Balances and ApplicationsAntonio HernandezNo ratings yet
- Material Balance LectureDocument52 pagesMaterial Balance LectureAsh YehiaNo ratings yet
- CONTINUOUS DistillationDocument5 pagesCONTINUOUS DistillationNaseer SattarNo ratings yet
- 4, Material Balance ReactionDocument87 pages4, Material Balance ReactionDanang Präbowo100% (2)
- W4 L2 Control of Primary Particulates - Wall CollectorsDocument80 pagesW4 L2 Control of Primary Particulates - Wall CollectorsZafirahAhmadFauziNo ratings yet
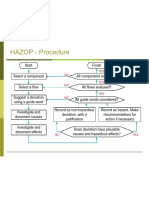
- Example: HAZOP Analysis of A Fueling Terminal For A BargeDocument4 pagesExample: HAZOP Analysis of A Fueling Terminal For A BargeBharat LalNo ratings yet
- Files 2-Experiments Homogenuous Batch ReactorDocument6 pagesFiles 2-Experiments Homogenuous Batch ReactorS M AseemNo ratings yet
- ChemicalDocument232 pagesChemicalhendra93No ratings yet
- Tubular Flow ReactorDocument21 pagesTubular Flow ReactorSabrinaNo ratings yet
- PROP6020 PDocument20 pagesPROP6020 PlukeneerNo ratings yet
- Unit Operation Lab ReportDocument4 pagesUnit Operation Lab ReportJosephine Wong Sian CheeNo ratings yet
- Plug Flow ReactorDocument16 pagesPlug Flow ReactorN Afiqah RazakNo ratings yet
- Iso Batch ReactorDocument10 pagesIso Batch ReactorSakethBharadwajNo ratings yet
- Intro Heat Exchanger Process ControlDocument3 pagesIntro Heat Exchanger Process ControlAimi AthirahNo ratings yet
- Hazop Distillation ColumnDocument4 pagesHazop Distillation ColumnMUSNo ratings yet
- Piper AlphaDocument6 pagesPiper AlphaAhmed Eldeeb100% (1)
- Ice Cream Manufacturing and Distribution CompanyDocument4 pagesIce Cream Manufacturing and Distribution Companypushpamali0% (1)
- Ethyl Acetate Kinetics 2011Document9 pagesEthyl Acetate Kinetics 2011Paola moreno100% (1)
- Gas Absorption and Gas StrippingDocument14 pagesGas Absorption and Gas StrippingEK63No ratings yet
- HTO Lab Manual Fall-18 PDFDocument52 pagesHTO Lab Manual Fall-18 PDFhumair khaliqNo ratings yet
- Soap Lab Report by ADDocument12 pagesSoap Lab Report by ADAD50% (4)
- Plug Flow ReactorDocument16 pagesPlug Flow Reactormirdza94No ratings yet
- Experiment 1 - DiffusionDocument31 pagesExperiment 1 - DiffusionPatricia Ann Mae0% (1)
- Boiling Heat Transfer ExpDocument22 pagesBoiling Heat Transfer ExpMahesh Vp0% (1)
- Muhammad Khaleel C.V - Senior Process Operation Engineer PDFDocument4 pagesMuhammad Khaleel C.V - Senior Process Operation Engineer PDFMuhammad KhaleelNo ratings yet
- Chapter 3 - Stage and Continuous Gas-Liquid Separation ProcessesDocument46 pagesChapter 3 - Stage and Continuous Gas-Liquid Separation Processesomarfhassan0% (1)
- Report Compiled 1Document14 pagesReport Compiled 1Opeyemi KehindeNo ratings yet
- Tubular Flow Reactor Sample UiTM Lab ReportDocument20 pagesTubular Flow Reactor Sample UiTM Lab ReportNur AqilahNo ratings yet
- Chemical Process Principles (CLB10904) : Chapter 2 Material Balance: (PART 1)Document46 pagesChemical Process Principles (CLB10904) : Chapter 2 Material Balance: (PART 1)FATMIENo ratings yet
- Lab Manual Gas Pressure Process ControlDocument10 pagesLab Manual Gas Pressure Process ControlAziemah AulanNo ratings yet
- Slides - Plug Flow Reactor (2018)Document36 pagesSlides - Plug Flow Reactor (2018)Meireza Ajeng Pratiwi100% (1)
- Hazard and Operability Studies (HAZOP) 2Document15 pagesHazard and Operability Studies (HAZOP) 2jeevanantham 5846No ratings yet
- Process Control and HazopDocument12 pagesProcess Control and HazopCosmin FloreaNo ratings yet
- CE 620 Liquid-Liquid Extraction Unit: Instruction ManualDocument39 pagesCE 620 Liquid-Liquid Extraction Unit: Instruction ManualRiccardo Vianello100% (1)
- Mass Balance AnalysisDocument11 pagesMass Balance AnalysisSatish GanesanNo ratings yet
- Drying: Merry Jessah S. TorresDocument6 pagesDrying: Merry Jessah S. TorresFrancis Val FranciscoNo ratings yet
- Mass Transfer: The Gate CoachDocument28 pagesMass Transfer: The Gate CoachSandeep CharanNo ratings yet
- 402 - Mass BalanceDocument5 pages402 - Mass BalanceSajesh S KumarNo ratings yet
- Lec 2 Fundamentals of Material BalancesDocument67 pagesLec 2 Fundamentals of Material BalancesEli EliNo ratings yet
- Lec 2 Fundamentals of Material BalancesDocument73 pagesLec 2 Fundamentals of Material Balancesjan gastiloNo ratings yet
- Kinetic Evaluation of Ethyl Acetate Production For Local Alimentary Solvents ProductionDocument7 pagesKinetic Evaluation of Ethyl Acetate Production For Local Alimentary Solvents ProductionDiego Nicolas ManceraNo ratings yet
- Lecture 1 - IntroductionDocument20 pagesLecture 1 - IntroductionDavid Rivera Arjona100% (1)
- Innovaya Visual EstimatingDocument2 pagesInnovaya Visual EstimatingJoshua JohnsonNo ratings yet
- Data Sartocell Filter Modules For F+B SR-2011-eDocument2 pagesData Sartocell Filter Modules For F+B SR-2011-eJoshua JohnsonNo ratings yet
- Medium Standard Air VolumeDocument3 pagesMedium Standard Air VolumeJoshua JohnsonNo ratings yet
- Malindo Air - Smarter Way To TravelDocument4 pagesMalindo Air - Smarter Way To TravelJoshua JohnsonNo ratings yet
- Annex 3 AnzcepDocument21 pagesAnnex 3 AnzcepJoshua JohnsonNo ratings yet
- 05 Chapter 1Document15 pages05 Chapter 1Joshua JohnsonNo ratings yet
- Additive 484Document3 pagesAdditive 484Joshua JohnsonNo ratings yet
- PDFDocument63 pagesPDFJoshua JohnsonNo ratings yet
- Basic Principles of GMP: PremisesDocument39 pagesBasic Principles of GMP: PremisesJoshua JohnsonNo ratings yet
- Office Politics: Are You A Player?Document2 pagesOffice Politics: Are You A Player?Joshua JohnsonNo ratings yet
- Tank Insulation CalculatorDocument4 pagesTank Insulation CalculatorJoshua JohnsonNo ratings yet
- Mechine Readable PassportDocument1 pageMechine Readable PassportJoshua JohnsonNo ratings yet
- CPL 40 CPL 40 20Document23 pagesCPL 40 CPL 40 20Joshua JohnsonNo ratings yet
- Wiper Seal Data Sheet A02 B 11782 1 enDocument2 pagesWiper Seal Data Sheet A02 B 11782 1 enghanNo ratings yet
- Fouling FactorsDocument6 pagesFouling FactorsDayo IdowuNo ratings yet
- Domestic Production and Foreign Trade The American Capital Position Re-ExaminedDocument23 pagesDomestic Production and Foreign Trade The American Capital Position Re-ExaminedKevin Mcdonald100% (2)
- Mineral FibrelDocument19 pagesMineral FibrelnitishkohliNo ratings yet
- Flexural Stress Block Development For Polypropylene Hybrid Fibre Reinforced ConcreteDocument15 pagesFlexural Stress Block Development For Polypropylene Hybrid Fibre Reinforced ConcreteAjitpal Singh0% (1)
- ESAB Transportation Catalogue 201181132250 20118916190 PDFDocument68 pagesESAB Transportation Catalogue 201181132250 20118916190 PDFcengizarda1979No ratings yet
- John M Nathanael D, Festo Didactic: M711 - Fundamentals of MechatronicsDocument65 pagesJohn M Nathanael D, Festo Didactic: M711 - Fundamentals of MechatronicsPak Em-emNo ratings yet
- SloanDocument30 pagesSloanKritika AhujaNo ratings yet
- Cored Wires - ESAB - OK TubrodDocument87 pagesCored Wires - ESAB - OK TubrodElias KapaNo ratings yet
- Specification For Bituminous MacadamDocument13 pagesSpecification For Bituminous MacadamtdlongvraNo ratings yet
- Sportzentrum Mulimatti 2Document15 pagesSportzentrum Mulimatti 2mi biNo ratings yet
- Phase DiagramDocument5 pagesPhase DiagramBunnimit panyacheewathonNo ratings yet
- Egr - Procesos de Manufactura - 2.1Document11 pagesEgr - Procesos de Manufactura - 2.1Eleazar GarciaNo ratings yet
- 2005 Prevost PresentationDocument70 pages2005 Prevost Presentationtaufiqishak09No ratings yet
- CBT 22Document16 pagesCBT 22SUNIL RAJPUTNo ratings yet
- Ruanta C-12 TDSDocument1 pageRuanta C-12 TDSm daneshpourNo ratings yet
- Schott Borofloat Technical Data Sheet EnglishDocument1 pageSchott Borofloat Technical Data Sheet Englishlilian.agenaisNo ratings yet
- Ce6302 Notes Rejinpaul - 2 PDFDocument49 pagesCe6302 Notes Rejinpaul - 2 PDFSekar Dinesh50% (2)
- Mat Sci Engg PresentDocument39 pagesMat Sci Engg PresentH Janardan PrabhuNo ratings yet
- Eddy Current Testing ApplicationsDocument21 pagesEddy Current Testing ApplicationsvibinkumarsNo ratings yet
- Cantilever Tutorial For COMSOLDocument26 pagesCantilever Tutorial For COMSOLgpendharkarNo ratings yet
- Development of A Continuous Microchannel CrystallizerDocument4 pagesDevelopment of A Continuous Microchannel CrystallizerchenabeelNo ratings yet
- Doors and Wall Partitions 2021 2022 It en Glas Italia 0 Cat150e611fDocument289 pagesDoors and Wall Partitions 2021 2022 It en Glas Italia 0 Cat150e611fNihal JafarNo ratings yet
- Taylor Power SystemsDocument4 pagesTaylor Power SystemsTeresa CarterNo ratings yet
- Bidsheet - SMO CS WUR Earthwork General Package 4 - FINAL MITRADocument8 pagesBidsheet - SMO CS WUR Earthwork General Package 4 - FINAL MITRAbara laksaniNo ratings yet
- Arcelormittal Dofasco Scrap Specifications and Requirements: Revision Date: January 2017Document37 pagesArcelormittal Dofasco Scrap Specifications and Requirements: Revision Date: January 2017Tanveer us zamanNo ratings yet
- Suspencion 930E KOMATSUDocument37 pagesSuspencion 930E KOMATSUBryan Rafael Coronado Pinto100% (1)
- Data Sheet Hipotronics 100HTV (Redes Energizadas)Document2 pagesData Sheet Hipotronics 100HTV (Redes Energizadas)jonathans2701No ratings yet
- Appendix - Final Report - KAMULIDocument26 pagesAppendix - Final Report - KAMULIOjullaIsaacNo ratings yet