Professional Documents
Culture Documents
02-2 Water-TS Diagram PDF
02-2 Water-TS Diagram PDF
Uploaded by
Gerald RahanraCopyright:
Available Formats
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5820)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Process Design ConditionsDocument7 pagesProcess Design ConditionsGerald RahanraNo ratings yet
- Tugas 10 23020018Document9 pagesTugas 10 23020018Gerald RahanraNo ratings yet
- Pra-Rancangan Stripping Section Dan Furnace Pada Kerosene Hydrotreating Unit Di Kilang Tuban PT. PertaminaDocument7 pagesPra-Rancangan Stripping Section Dan Furnace Pada Kerosene Hydrotreating Unit Di Kilang Tuban PT. PertaminaGerald RahanraNo ratings yet
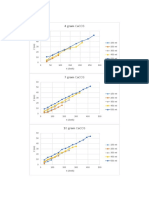
- 4 Gram Caco3: Grafik Z Vs TDocument4 pages4 Gram Caco3: Grafik Z Vs TGerald RahanraNo ratings yet
- Pinch Table AlgorithmDocument3 pagesPinch Table AlgorithmGerald RahanraNo ratings yet
- Process Design ConditionsDocument8 pagesProcess Design ConditionsGerald RahanraNo ratings yet
- 4 Gram Caco3: Grafik Z Vs TDocument3 pages4 Gram Caco3: Grafik Z Vs TGerald RahanraNo ratings yet
- Onliner Intensive SBMPTN, Friday, 6 June 2014 (English) by Agustini Choose The Best Answer! Text 1 For No. 1 - 5Document5 pagesOnliner Intensive SBMPTN, Friday, 6 June 2014 (English) by Agustini Choose The Best Answer! Text 1 For No. 1 - 5Gerald RahanraNo ratings yet
02-2 Water-TS Diagram PDF
02-2 Water-TS Diagram PDF
Uploaded by
Gerald RahanraOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
02-2 Water-TS Diagram PDF
02-2 Water-TS Diagram PDF
Uploaded by
Gerald RahanraCopyright:
Available Formats
5
9
h = 5000 kJ/kg
50
00
550
0
10
1200
1100
5000
4500
1100
4900
4800
kg/m 3
300 k
g/m 3
4200
4000
or
24
00
h=
00
26
00
k J/k
Qu
70
0k
a lit
y=
5
Entropy, kJ/kg K
0.0
3k
g/m 3
0.01
kg/m 3
0.1
kg/m 3
200
100
2650
h = 2600
2650 kJ/kg
2550
90 %
300
2800
J/ k
20 0
80%
3100
2900
400
3200
3000
8
6
4
3
2
1.5
1.0
va
p
3300
De n
sity
=
22
te d
500
3400
0.
0 006
0. .004
0
0. 03
00
2
00
60%
50%
60 0
40%
00
00
20
ra
3500
0.8
0.6
0.4
0.3
0.2
0.1
5
0.1
18
16
00
%
20
14
1 20
1 00 0
80 0
=
ty
ali
Qu
h = 40
100
%
10
ate
tur
Sa
30
%
0
100
200
Sa
tu
id
iqu
dl
40
30
0
140
0
120
20
15
10
0
10
80 0
6
1600
300
3600
0.3
kg/m 3
1800
400
600
3700
0.0
0.08
6
0.
0
0.04
3
0.0
0.02
15
0.0
08
kJ/
2200 kg
h=
0
0
0
2
3800
1 kg/m 3
2400
500
30 kg
/m 3
10
00
0
80
0
60
0
50
0
40 0
35 00
3 0
25 0
20
0
15
2600
700
3900
3 kg/m 3
600
800
4100
3000
328200
0
0
4300
100 k
g/m 3
700
900
4400
10 kg
/m 3
Den
sity
=
3600
3200
P=
Temperature, C
4500
3800
3400
800
1000
4600
4000
300
20 00 b
150000 ar
100 00
80000
0
50
4 00 6000
30000
0
20 0
15 00
00
900
4700
0
10
FIGURE A9
T-s diagram for water.
Copyright 1984. From NBS/NRC Steam Tables/1 by Lester Haar, John S. Gallagher, and George S. Kell. Reproduced by permission of Routledge/Taylor & Francis
Books, Inc.
Thermodynamics
h = 4200 kJ/
kg
1000
cen84959_ch18-ap01.qxd 8/11/06 1:21 PM Page 926
926
0
1200
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5820)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Process Design ConditionsDocument7 pagesProcess Design ConditionsGerald RahanraNo ratings yet
- Tugas 10 23020018Document9 pagesTugas 10 23020018Gerald RahanraNo ratings yet
- Pra-Rancangan Stripping Section Dan Furnace Pada Kerosene Hydrotreating Unit Di Kilang Tuban PT. PertaminaDocument7 pagesPra-Rancangan Stripping Section Dan Furnace Pada Kerosene Hydrotreating Unit Di Kilang Tuban PT. PertaminaGerald RahanraNo ratings yet
- 4 Gram Caco3: Grafik Z Vs TDocument4 pages4 Gram Caco3: Grafik Z Vs TGerald RahanraNo ratings yet
- Pinch Table AlgorithmDocument3 pagesPinch Table AlgorithmGerald RahanraNo ratings yet
- Process Design ConditionsDocument8 pagesProcess Design ConditionsGerald RahanraNo ratings yet
- 4 Gram Caco3: Grafik Z Vs TDocument3 pages4 Gram Caco3: Grafik Z Vs TGerald RahanraNo ratings yet
- Onliner Intensive SBMPTN, Friday, 6 June 2014 (English) by Agustini Choose The Best Answer! Text 1 For No. 1 - 5Document5 pagesOnliner Intensive SBMPTN, Friday, 6 June 2014 (English) by Agustini Choose The Best Answer! Text 1 For No. 1 - 5Gerald RahanraNo ratings yet