Professional Documents
Culture Documents
Fukuyama Group - Group Meeting Problems 08/08/2011: T-Buok, N-Buli N-Buli (1.1 Eq)
Fukuyama Group - Group Meeting Problems 08/08/2011: T-Buok, N-Buli N-Buli (1.1 Eq)
Uploaded by
Anonymous rhUNqC1s0Copyright:
Available Formats
You might also like
- Fukuyama Group - Group Meeting Problems 2/15/2012: Et Si Siet O D Thf-Hmpa - 78 °C 80% Et Si Siet Oh DDocument1 pageFukuyama Group - Group Meeting Problems 2/15/2012: Et Si Siet O D Thf-Hmpa - 78 °C 80% Et Si Siet Oh DAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 11/10/2012: PH BF KDocument3 pagesFukuyama Group - Group Meeting Problems 11/10/2012: PH BF KAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 01/11/2013: T-BuliDocument4 pagesFukuyama Group - Group Meeting Problems 01/11/2013: T-BuliAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 07/17/2011: Low Yield Under Basic ConditionDocument3 pagesFukuyama Group - Group Meeting Problems 07/17/2011: Low Yield Under Basic ConditionAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 10/06/2012: J. Rodriguez Et Al., Org. Lett., 12, 4212 (2010)Document3 pagesFukuyama Group - Group Meeting Problems 10/06/2012: J. Rodriguez Et Al., Org. Lett., 12, 4212 (2010)Anonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 08/01/2012: Otes MeDocument3 pagesFukuyama Group - Group Meeting Problems 08/01/2012: Otes MeAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 1/17/2012: Bicyclic CompoundDocument1 pageFukuyama Group - Group Meeting Problems 1/17/2012: Bicyclic CompoundAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 09/07/2011: O N O Me MeDocument2 pagesFukuyama Group - Group Meeting Problems 09/07/2011: O N O Me MeAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 06/02/2010: O P N O Ome OmeDocument2 pagesFukuyama Group - Group Meeting Problems 06/02/2010: O P N O Ome OmeAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 10/01/2011: N-Buli (2 Eq), - 20 °CDocument2 pagesFukuyama Group - Group Meeting Problems 10/01/2011: N-Buli (2 Eq), - 20 °CAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 09/05/2012: P-Tsoh (Cat.)Document2 pagesFukuyama Group - Group Meeting Problems 09/05/2012: P-Tsoh (Cat.)Anonymous rhUNqC1s0No ratings yet
- 1987 Mayr - Acid - and Base-Catalyzed Ring-Opening Reactions or A Sterically Hindered Epoxide PDFDocument3 pages1987 Mayr - Acid - and Base-Catalyzed Ring-Opening Reactions or A Sterically Hindered Epoxide PDFHernán AstudilloNo ratings yet
- An Efficient Synthesis of Racemic TolterodineDocument2 pagesAn Efficient Synthesis of Racemic TolterodineJignesh TrivediNo ratings yet
- OH O O: Griffiths, Russell Jon, PCT Int. Appl., 2013124682, 29 Aug 2013Document3 pagesOH O O: Griffiths, Russell Jon, PCT Int. Appl., 2013124682, 29 Aug 2013kasun1237459No ratings yet
- Fukuyama Group - Group Meeting Problems 12/12/2012: CO Me NDocument2 pagesFukuyama Group - Group Meeting Problems 12/12/2012: CO Me NAnonymous rhUNqC1s0No ratings yet
- Lysergic Kurokawa PDFDocument15 pagesLysergic Kurokawa PDFBrandon TimmNo ratings yet
- ANTHONY CRASTO-Brevetoxin SynthesisDocument58 pagesANTHONY CRASTO-Brevetoxin SynthesisAnthony Melvin Crasto Ph.DNo ratings yet
- Synthesis, Characterization, and Organic Chemistry of An Edge-Bridged Half-Open TitanoceneDocument8 pagesSynthesis, Characterization, and Organic Chemistry of An Edge-Bridged Half-Open TitanoceneANTONIO C.MNo ratings yet
- Synthesis of Schiff Bases by Aromatic Amine Condensation With 3,3 - Bithiophenes-2,2 and 4,4 - DicarbaldehydesDocument5 pagesSynthesis of Schiff Bases by Aromatic Amine Condensation With 3,3 - Bithiophenes-2,2 and 4,4 - DicarbaldehydesYsabel Huaccallo AguilarNo ratings yet
- Tetrahedron Letters Volume 40 Issue 5 1999 (Doi 10.1016/s0040-4039 (98) 02540-4) Francisco JoséRomero-Salguero Jean-Marie Lehn - Synthesis of Multitopic Bidentate Ligands Based On Alternating PyDocument4 pagesTetrahedron Letters Volume 40 Issue 5 1999 (Doi 10.1016/s0040-4039 (98) 02540-4) Francisco JoséRomero-Salguero Jean-Marie Lehn - Synthesis of Multitopic Bidentate Ligands Based On Alternating PyJoakin BahamondesNo ratings yet
- 24 1 21-4附件1Document28 pages24 1 21-4附件12238505229No ratings yet
- Ja0449269 Si 001Document17 pagesJa0449269 Si 001Origamist KryaNo ratings yet
- Química OrganicaDocument6 pagesQuímica OrganicaVllsSNo ratings yet
- 07.Mmc ExperimentalDocument14 pages07.Mmc ExperimentalNurul Putry SyahrulNo ratings yet
- jm9b01579 Si 001Document36 pagesjm9b01579 Si 001Jagjeet GujralNo ratings yet
- 1 s2.0 S0022286021011455 mmc1Document49 pages1 s2.0 S0022286021011455 mmc1jipir64332No ratings yet
- Rhodium-Catalyzed Triarylphosphine Synthesis Via Cross-Coupling ofDocument43 pagesRhodium-Catalyzed Triarylphosphine Synthesis Via Cross-Coupling ofGabriela ArequipaNo ratings yet
- Wjoc 2 1 1 PDFDocument8 pagesWjoc 2 1 1 PDFWalid Ebid ElgammalNo ratings yet
- Ol901892s Si 001Document80 pagesOl901892s Si 001RenataSucupiraNo ratings yet
- Differential Recognition of Deacetylated PNAG Oligosaccharides by A Biofilm Degrading GlycosidaseDocument52 pagesDifferential Recognition of Deacetylated PNAG Oligosaccharides by A Biofilm Degrading GlycosidaseLuís MiguelNo ratings yet
- Ol048777a Si 001Document14 pagesOl048777a Si 001Aitor LezamaNo ratings yet
- Reaxys Anonymous 20131108 024356 919Document80 pagesReaxys Anonymous 20131108 024356 919ebi1364No ratings yet
- Cooper1979 PDFDocument3 pagesCooper1979 PDFJ Venkat RamanNo ratings yet
- Ja802896n Si 001Document22 pagesJa802896n Si 001ArjunvrstlNo ratings yet
- Chem 215 Myers: Birch ReductionDocument7 pagesChem 215 Myers: Birch ReductionPrasanna AndojuNo ratings yet
- jm901265h Si 001Document37 pagesjm901265h Si 001Quoc AnhNo ratings yet
- Amberlyst-15-Catalyzed Novel Synthesis of Quinoline Derivatives in Ionic LiquidDocument4 pagesAmberlyst-15-Catalyzed Novel Synthesis of Quinoline Derivatives in Ionic LiquidkamalnandreNo ratings yet
- Ol9011772 Si 001Document8 pagesOl9011772 Si 001James PerianayagamNo ratings yet
- 1990 Annonaceous Acetogenins - A ReviewDocument42 pages1990 Annonaceous Acetogenins - A ReviewJuan PizanoNo ratings yet
- Preparation of Diethyl Malonate Adducts From Chalcone Analogs Containing A Thienyl RingDocument7 pagesPreparation of Diethyl Malonate Adducts From Chalcone Analogs Containing A Thienyl RingGabriel PekárekNo ratings yet
- Phenazine DerivateDocument37 pagesPhenazine Derivatelost6taNo ratings yet
- An Efficient Three Component Synthesis of 1H-Indazolo (2, 1-b) Phthalazine-Triones Catalyzed by Orthophosphoric Acid in Water-Phenol SystemDocument7 pagesAn Efficient Three Component Synthesis of 1H-Indazolo (2, 1-b) Phthalazine-Triones Catalyzed by Orthophosphoric Acid in Water-Phenol SystemreddymasumscNo ratings yet
- Eisch 1992Document4 pagesEisch 1992Natan FilippiNo ratings yet
- TLCT A 1900432 sm9470Document22 pagesTLCT A 1900432 sm9470Abdelghani HakimNo ratings yet
- Org Syn Collective Vol 9. Page 88Document4 pagesOrg Syn Collective Vol 9. Page 88rrgodboleNo ratings yet
- CCSC 2022 02418 File002Document63 pagesCCSC 2022 02418 File002migenyasuyoshiNo ratings yet
- SI A General Strategy For The Construction of Calyciphylline A Type Alkaloids Divergent Total Syntheses of Daphenylline and Himalensine ADocument46 pagesSI A General Strategy For The Construction of Calyciphylline A Type Alkaloids Divergent Total Syntheses of Daphenylline and Himalensine ADương Đức AnhNo ratings yet
- Synthesis of Carboxylic Acids, Esters, Alcohols and Ethers Containing A Tetrahydropyran Ring Derived From 6-Methyl-5-Hepten-2-OneDocument10 pagesSynthesis of Carboxylic Acids, Esters, Alcohols and Ethers Containing A Tetrahydropyran Ring Derived From 6-Methyl-5-Hepten-2-OnebiologiNo ratings yet
- Preview 8Document94 pagesPreview 8Rohan PrajapatiNo ratings yet
- Shekarchi 2008Document9 pagesShekarchi 2008jipir64332No ratings yet
- Ol403156r Si 001Document59 pagesOl403156r Si 001Màu Tím Purple LàNo ratings yet
- Triterpenes and Steroids From Semi-Mangrove Plant Hibiscus TiliaceusDocument3 pagesTriterpenes and Steroids From Semi-Mangrove Plant Hibiscus TiliaceusRidho Dhe HolmesNo ratings yet
- KT CH ActivationDocument36 pagesKT CH ActivationJayendra Pandit AhireNo ratings yet
- Coupling ReactionDocument7 pagesCoupling Reactionjulia2972003No ratings yet
- Experimental Section: 1 The The To The andDocument3 pagesExperimental Section: 1 The The To The andPrashant SinghNo ratings yet
- Journal of Organometallic Chemistry: Dominik Wechsler, Gabriele Schatte, Mark StradiottoDocument5 pagesJournal of Organometallic Chemistry: Dominik Wechsler, Gabriele Schatte, Mark StradiottoJoakin BahamondesNo ratings yet
- Supporting Information: Experimental Procedures and Analytical Data For New CompoundsDocument17 pagesSupporting Information: Experimental Procedures and Analytical Data For New CompoundsomansuNo ratings yet
- Fitoterapia: Dong Pei, Jun-Xi Liu, Duo-Long DiDocument6 pagesFitoterapia: Dong Pei, Jun-Xi Liu, Duo-Long DiDuong Pham QuangNo ratings yet
- Sulfur-Containing ReagentsFrom EverandSulfur-Containing ReagentsLeo A. PaquetteNo ratings yet
- Transport Processes in Chemically Reacting Flow SystemsFrom EverandTransport Processes in Chemically Reacting Flow SystemsRating: 5 out of 5 stars5/5 (1)
- 1986 - CHA - HAL - Thermodyn Prop C1-C4 Ideal GasDocument68 pages1986 - CHA - HAL - Thermodyn Prop C1-C4 Ideal GasAnonymous rhUNqC1s0No ratings yet
- Exam III Study GuideDocument2 pagesExam III Study GuideAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 1/17/2012: Bicyclic CompoundDocument1 pageFukuyama Group - Group Meeting Problems 1/17/2012: Bicyclic CompoundAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 09/05/2012: P-Tsoh (Cat.)Document2 pagesFukuyama Group - Group Meeting Problems 09/05/2012: P-Tsoh (Cat.)Anonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 01/11/2013: T-BuliDocument4 pagesFukuyama Group - Group Meeting Problems 01/11/2013: T-BuliAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 09/07/2011: O N O Me MeDocument2 pagesFukuyama Group - Group Meeting Problems 09/07/2011: O N O Me MeAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 07/17/2011: Low Yield Under Basic ConditionDocument3 pagesFukuyama Group - Group Meeting Problems 07/17/2011: Low Yield Under Basic ConditionAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 10/06/2012: J. Rodriguez Et Al., Org. Lett., 12, 4212 (2010)Document3 pagesFukuyama Group - Group Meeting Problems 10/06/2012: J. Rodriguez Et Al., Org. Lett., 12, 4212 (2010)Anonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 2/15/2012: Et Si Siet O D Thf-Hmpa - 78 °C 80% Et Si Siet Oh DDocument1 pageFukuyama Group - Group Meeting Problems 2/15/2012: Et Si Siet O D Thf-Hmpa - 78 °C 80% Et Si Siet Oh DAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 11/10/2012: PH BF KDocument3 pagesFukuyama Group - Group Meeting Problems 11/10/2012: PH BF KAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 12/12/2012: CO Me NDocument2 pagesFukuyama Group - Group Meeting Problems 12/12/2012: CO Me NAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 10/01/2011: N-Buli (2 Eq), - 20 °CDocument2 pagesFukuyama Group - Group Meeting Problems 10/01/2011: N-Buli (2 Eq), - 20 °CAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 08/01/2012: Otes MeDocument3 pagesFukuyama Group - Group Meeting Problems 08/01/2012: Otes MeAnonymous rhUNqC1s0No ratings yet
- A 1204Document7 pagesA 1204Anonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 06/02/2010: O P N O Ome OmeDocument2 pagesFukuyama Group - Group Meeting Problems 06/02/2010: O P N O Ome OmeAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 12/11/2010: T-BuomeDocument1 pageFukuyama Group - Group Meeting Problems 12/11/2010: T-BuomeAnonymous rhUNqC1s0No ratings yet
Fukuyama Group - Group Meeting Problems 08/08/2011: T-Buok, N-Buli N-Buli (1.1 Eq)
Fukuyama Group - Group Meeting Problems 08/08/2011: T-Buok, N-Buli N-Buli (1.1 Eq)
Uploaded by
Anonymous rhUNqC1s0Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fukuyama Group - Group Meeting Problems 08/08/2011: T-Buok, N-Buli N-Buli (1.1 Eq)
Fukuyama Group - Group Meeting Problems 08/08/2011: T-Buok, N-Buli N-Buli (1.1 Eq)
Uploaded by
Anonymous rhUNqC1s0Copyright:
Available Formats
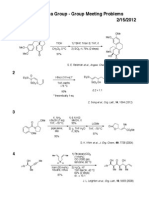
Fukuyama Group - Group Meeting Problems
08/08/2011
1
OTs
O N
S
Me
1) n-BuLi, THF
50 C to rt;
MeI, 78 C, 94%
t-BuOK, n-BuLi
n-BuLi (1.1 eq)
[D8]-THF, 78 C;
H
N
rt, 24 h;
H2O
2) n-BuLi, THF
50 C to rt;
MeI, 78 C, 54%
THF, 78 C to rt
66%
84%
Me
Me
H.-J. Gais et al., Synthesis, 919 (1998)
H.-J. Gais et al., Eur. J. Org. Chem., 2431 (2011)
2
SPh
(phenylthio)acetic acid
KHMDS
TMSCl, pyridine
DEAD, PPh3
MeO
Zwitterionic
Compound
THF, toluene
78 to 60 C
THF, 0 C
HO
81%
57%
Me
OH
MeOH
40 C
77%
T. Nishikawa et al., J. Org. Chem., 76, 6942 (2011)
3
Ph
HN
Ph
(COCl)2, CH2Cl2, rt;
C18H15NO3
FeCl3, 10 C to rt
Me
H2SO4
MeOH, reflux
88% (2 steps)
Me
R. D. Larsen et al., J. Org. Chem., 56, 6034 (1991)
4
Si
O
O
t-Bu
t-Bu
AgOCOCF3 (1 mol%)
toluene, rt
95%
1) LiAlH4
THF, reflux
2) Ph(CH3)2CO2H
KH, CsF
NMP-THF, rt
87% (2 steps)
OH
OH
K. A. Woerpel et al., J. Am. Chem. Soc., 127, 2046 (2005)
Fukuyama Group - Group Meeting Problems
08/21/2011
1
Me
CO2Et
OH
Ph
Ph
AgSbF6 (5 mol%)
CH2Cl2, rt;
DABCO (cat.)
Me
(Ph3P)AuCl (5 mol%)
PhNH2, 38 C
benzene, reflux
89%
CO2Et
Me
N
Ph
75%
A. Saito, Y. Hanzawa et al., Org. Lett., 12, 372 (2010)
S. F. Kirsch et al., Org. Lett., 8, 2151 (2006)
SEt
O
TFAA, Et3N
CH2Cl2, 78 C;
MeO
EtS
CO2Me
MeO
MeO
BF3OEt2
78 C to rt;
reflux
MeO
CO2Me
MeO
83%
A. Padwa et al., J. Org. Chem., 63, 1144 (1998)
3
CHBr3
Ph
Br
CH2Cl2-NaOH aq.
rt
1) MeLi, Et2O
78 to 50 C;
tBuLi, 78 C;
A, rt, 75%
2)
TIPS
Ph
Br
TIPS
N
H11C6
Bu4HSO4, NaOH
toluene, rt, 62%
H11C6
Ts
Ts
P. Wipf et al., Angew. Chem. Int. Ed., 45, 4172 (2006)
OPh
PhO P N3
O
Me3SiCH2MgCl
Et2O, 10 to 0C;
reagent A
H2O, 15 C
6770%
A, InCl3 (2 mol%)
CH2Cl2, rt;
evap.;
O
H
A, n-BuLi, THF
78 C to rt
65%
1H
NMR (CDCl3, 500 MHz)
= 7.32 (t, J = 7.7 Hz, 2H)
PtCl2 (5 mol%)
7.23 (d, J = 7.2 Hz, 3H)
2.93 (m, 4H)
CH2Cl2, 50 C
0.83 (s, 2H)
95%
0.17 (s, 9H)
SiMe3
T. Shioiri et al., Org. Synth., Coll. Vol. 8, 612 (1990)
D. Lee et al., J. Am. Chem. Soc., 132, 6640 (2010)
D. Lee et al., J. Am. Chem. Soc., 133, 12964 (2011)
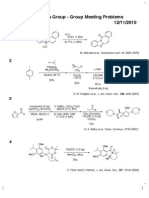
Fukuyama Group - Group Meeting Problems
08/31/2011
1
S2Cl2 (10 eq)
DABCO (8 eq)
chlorobenzene, rt;
N
HCO2H (excess)
reflux
N
S
42%
C. W. Rees et al., J. Org. Chem., 63, 2189 (1998)
S
S
1) n-BuLi, nitroethene
THF, 78 C
49%
1) PhI(OCOCF3)2
MeOH, rt
44%
2) Boc2O, DMAP
toluene, 90 C
80%
2) Raney Ni, B(OH)3
MeOH, H2O, 25 C
70%
3) CSA, DCE, rt, 68%
HO
OMe
H. Nussbaumer et al., Synlett., 13, 1966 (2010)
MeO
1)
N
NH2
Ph
CH2Cl2, rt
2) NaH (4 eq)
MeI (2 eq)
THF, 0 C to rt
34%
1H-NMR
(CDCl3, 300 MHZ)
= 7.11-6.48 (m, 9H)
5.17 (q, 1H, J = 7.0 Hz)
3.57 (s, 3H)
2.99 (s, 3H)
2.78 (s, 3H)
1.34 (d, 3H, J = 7.0 Hz)
1) i-PrLi
toluene, 40 C;
DMPU
40 C to rt
54%
OMe
MeHN
2) n-BuOH, reflux
70%
i-Pr
Ph
Me
J. Clayden et al., J. Am. Chem. Soc., 132, 6624 (2010)
4
O2, TPP
h
CH2Cl2
78 C;
KO2CN NCO2K
AcOH
78 to 40 C
H
CCl4
60-80 C
52% (2 steps)
I. Erden et al., Angew. Chem. Int. Ed., 36, 1516 (1997)
You might also like
- Fukuyama Group - Group Meeting Problems 2/15/2012: Et Si Siet O D Thf-Hmpa - 78 °C 80% Et Si Siet Oh DDocument1 pageFukuyama Group - Group Meeting Problems 2/15/2012: Et Si Siet O D Thf-Hmpa - 78 °C 80% Et Si Siet Oh DAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 11/10/2012: PH BF KDocument3 pagesFukuyama Group - Group Meeting Problems 11/10/2012: PH BF KAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 01/11/2013: T-BuliDocument4 pagesFukuyama Group - Group Meeting Problems 01/11/2013: T-BuliAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 07/17/2011: Low Yield Under Basic ConditionDocument3 pagesFukuyama Group - Group Meeting Problems 07/17/2011: Low Yield Under Basic ConditionAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 10/06/2012: J. Rodriguez Et Al., Org. Lett., 12, 4212 (2010)Document3 pagesFukuyama Group - Group Meeting Problems 10/06/2012: J. Rodriguez Et Al., Org. Lett., 12, 4212 (2010)Anonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 08/01/2012: Otes MeDocument3 pagesFukuyama Group - Group Meeting Problems 08/01/2012: Otes MeAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 1/17/2012: Bicyclic CompoundDocument1 pageFukuyama Group - Group Meeting Problems 1/17/2012: Bicyclic CompoundAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 09/07/2011: O N O Me MeDocument2 pagesFukuyama Group - Group Meeting Problems 09/07/2011: O N O Me MeAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 06/02/2010: O P N O Ome OmeDocument2 pagesFukuyama Group - Group Meeting Problems 06/02/2010: O P N O Ome OmeAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 10/01/2011: N-Buli (2 Eq), - 20 °CDocument2 pagesFukuyama Group - Group Meeting Problems 10/01/2011: N-Buli (2 Eq), - 20 °CAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 09/05/2012: P-Tsoh (Cat.)Document2 pagesFukuyama Group - Group Meeting Problems 09/05/2012: P-Tsoh (Cat.)Anonymous rhUNqC1s0No ratings yet
- 1987 Mayr - Acid - and Base-Catalyzed Ring-Opening Reactions or A Sterically Hindered Epoxide PDFDocument3 pages1987 Mayr - Acid - and Base-Catalyzed Ring-Opening Reactions or A Sterically Hindered Epoxide PDFHernán AstudilloNo ratings yet
- An Efficient Synthesis of Racemic TolterodineDocument2 pagesAn Efficient Synthesis of Racemic TolterodineJignesh TrivediNo ratings yet
- OH O O: Griffiths, Russell Jon, PCT Int. Appl., 2013124682, 29 Aug 2013Document3 pagesOH O O: Griffiths, Russell Jon, PCT Int. Appl., 2013124682, 29 Aug 2013kasun1237459No ratings yet
- Fukuyama Group - Group Meeting Problems 12/12/2012: CO Me NDocument2 pagesFukuyama Group - Group Meeting Problems 12/12/2012: CO Me NAnonymous rhUNqC1s0No ratings yet
- Lysergic Kurokawa PDFDocument15 pagesLysergic Kurokawa PDFBrandon TimmNo ratings yet
- ANTHONY CRASTO-Brevetoxin SynthesisDocument58 pagesANTHONY CRASTO-Brevetoxin SynthesisAnthony Melvin Crasto Ph.DNo ratings yet
- Synthesis, Characterization, and Organic Chemistry of An Edge-Bridged Half-Open TitanoceneDocument8 pagesSynthesis, Characterization, and Organic Chemistry of An Edge-Bridged Half-Open TitanoceneANTONIO C.MNo ratings yet
- Synthesis of Schiff Bases by Aromatic Amine Condensation With 3,3 - Bithiophenes-2,2 and 4,4 - DicarbaldehydesDocument5 pagesSynthesis of Schiff Bases by Aromatic Amine Condensation With 3,3 - Bithiophenes-2,2 and 4,4 - DicarbaldehydesYsabel Huaccallo AguilarNo ratings yet
- Tetrahedron Letters Volume 40 Issue 5 1999 (Doi 10.1016/s0040-4039 (98) 02540-4) Francisco JoséRomero-Salguero Jean-Marie Lehn - Synthesis of Multitopic Bidentate Ligands Based On Alternating PyDocument4 pagesTetrahedron Letters Volume 40 Issue 5 1999 (Doi 10.1016/s0040-4039 (98) 02540-4) Francisco JoséRomero-Salguero Jean-Marie Lehn - Synthesis of Multitopic Bidentate Ligands Based On Alternating PyJoakin BahamondesNo ratings yet
- 24 1 21-4附件1Document28 pages24 1 21-4附件12238505229No ratings yet
- Ja0449269 Si 001Document17 pagesJa0449269 Si 001Origamist KryaNo ratings yet
- Química OrganicaDocument6 pagesQuímica OrganicaVllsSNo ratings yet
- 07.Mmc ExperimentalDocument14 pages07.Mmc ExperimentalNurul Putry SyahrulNo ratings yet
- jm9b01579 Si 001Document36 pagesjm9b01579 Si 001Jagjeet GujralNo ratings yet
- 1 s2.0 S0022286021011455 mmc1Document49 pages1 s2.0 S0022286021011455 mmc1jipir64332No ratings yet
- Rhodium-Catalyzed Triarylphosphine Synthesis Via Cross-Coupling ofDocument43 pagesRhodium-Catalyzed Triarylphosphine Synthesis Via Cross-Coupling ofGabriela ArequipaNo ratings yet
- Wjoc 2 1 1 PDFDocument8 pagesWjoc 2 1 1 PDFWalid Ebid ElgammalNo ratings yet
- Ol901892s Si 001Document80 pagesOl901892s Si 001RenataSucupiraNo ratings yet
- Differential Recognition of Deacetylated PNAG Oligosaccharides by A Biofilm Degrading GlycosidaseDocument52 pagesDifferential Recognition of Deacetylated PNAG Oligosaccharides by A Biofilm Degrading GlycosidaseLuís MiguelNo ratings yet
- Ol048777a Si 001Document14 pagesOl048777a Si 001Aitor LezamaNo ratings yet
- Reaxys Anonymous 20131108 024356 919Document80 pagesReaxys Anonymous 20131108 024356 919ebi1364No ratings yet
- Cooper1979 PDFDocument3 pagesCooper1979 PDFJ Venkat RamanNo ratings yet
- Ja802896n Si 001Document22 pagesJa802896n Si 001ArjunvrstlNo ratings yet
- Chem 215 Myers: Birch ReductionDocument7 pagesChem 215 Myers: Birch ReductionPrasanna AndojuNo ratings yet
- jm901265h Si 001Document37 pagesjm901265h Si 001Quoc AnhNo ratings yet
- Amberlyst-15-Catalyzed Novel Synthesis of Quinoline Derivatives in Ionic LiquidDocument4 pagesAmberlyst-15-Catalyzed Novel Synthesis of Quinoline Derivatives in Ionic LiquidkamalnandreNo ratings yet
- Ol9011772 Si 001Document8 pagesOl9011772 Si 001James PerianayagamNo ratings yet
- 1990 Annonaceous Acetogenins - A ReviewDocument42 pages1990 Annonaceous Acetogenins - A ReviewJuan PizanoNo ratings yet
- Preparation of Diethyl Malonate Adducts From Chalcone Analogs Containing A Thienyl RingDocument7 pagesPreparation of Diethyl Malonate Adducts From Chalcone Analogs Containing A Thienyl RingGabriel PekárekNo ratings yet
- Phenazine DerivateDocument37 pagesPhenazine Derivatelost6taNo ratings yet
- An Efficient Three Component Synthesis of 1H-Indazolo (2, 1-b) Phthalazine-Triones Catalyzed by Orthophosphoric Acid in Water-Phenol SystemDocument7 pagesAn Efficient Three Component Synthesis of 1H-Indazolo (2, 1-b) Phthalazine-Triones Catalyzed by Orthophosphoric Acid in Water-Phenol SystemreddymasumscNo ratings yet
- Eisch 1992Document4 pagesEisch 1992Natan FilippiNo ratings yet
- TLCT A 1900432 sm9470Document22 pagesTLCT A 1900432 sm9470Abdelghani HakimNo ratings yet
- Org Syn Collective Vol 9. Page 88Document4 pagesOrg Syn Collective Vol 9. Page 88rrgodboleNo ratings yet
- CCSC 2022 02418 File002Document63 pagesCCSC 2022 02418 File002migenyasuyoshiNo ratings yet
- SI A General Strategy For The Construction of Calyciphylline A Type Alkaloids Divergent Total Syntheses of Daphenylline and Himalensine ADocument46 pagesSI A General Strategy For The Construction of Calyciphylline A Type Alkaloids Divergent Total Syntheses of Daphenylline and Himalensine ADương Đức AnhNo ratings yet
- Synthesis of Carboxylic Acids, Esters, Alcohols and Ethers Containing A Tetrahydropyran Ring Derived From 6-Methyl-5-Hepten-2-OneDocument10 pagesSynthesis of Carboxylic Acids, Esters, Alcohols and Ethers Containing A Tetrahydropyran Ring Derived From 6-Methyl-5-Hepten-2-OnebiologiNo ratings yet
- Preview 8Document94 pagesPreview 8Rohan PrajapatiNo ratings yet
- Shekarchi 2008Document9 pagesShekarchi 2008jipir64332No ratings yet
- Ol403156r Si 001Document59 pagesOl403156r Si 001Màu Tím Purple LàNo ratings yet
- Triterpenes and Steroids From Semi-Mangrove Plant Hibiscus TiliaceusDocument3 pagesTriterpenes and Steroids From Semi-Mangrove Plant Hibiscus TiliaceusRidho Dhe HolmesNo ratings yet
- KT CH ActivationDocument36 pagesKT CH ActivationJayendra Pandit AhireNo ratings yet
- Coupling ReactionDocument7 pagesCoupling Reactionjulia2972003No ratings yet
- Experimental Section: 1 The The To The andDocument3 pagesExperimental Section: 1 The The To The andPrashant SinghNo ratings yet
- Journal of Organometallic Chemistry: Dominik Wechsler, Gabriele Schatte, Mark StradiottoDocument5 pagesJournal of Organometallic Chemistry: Dominik Wechsler, Gabriele Schatte, Mark StradiottoJoakin BahamondesNo ratings yet
- Supporting Information: Experimental Procedures and Analytical Data For New CompoundsDocument17 pagesSupporting Information: Experimental Procedures and Analytical Data For New CompoundsomansuNo ratings yet
- Fitoterapia: Dong Pei, Jun-Xi Liu, Duo-Long DiDocument6 pagesFitoterapia: Dong Pei, Jun-Xi Liu, Duo-Long DiDuong Pham QuangNo ratings yet
- Sulfur-Containing ReagentsFrom EverandSulfur-Containing ReagentsLeo A. PaquetteNo ratings yet
- Transport Processes in Chemically Reacting Flow SystemsFrom EverandTransport Processes in Chemically Reacting Flow SystemsRating: 5 out of 5 stars5/5 (1)
- 1986 - CHA - HAL - Thermodyn Prop C1-C4 Ideal GasDocument68 pages1986 - CHA - HAL - Thermodyn Prop C1-C4 Ideal GasAnonymous rhUNqC1s0No ratings yet
- Exam III Study GuideDocument2 pagesExam III Study GuideAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 1/17/2012: Bicyclic CompoundDocument1 pageFukuyama Group - Group Meeting Problems 1/17/2012: Bicyclic CompoundAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 09/05/2012: P-Tsoh (Cat.)Document2 pagesFukuyama Group - Group Meeting Problems 09/05/2012: P-Tsoh (Cat.)Anonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 01/11/2013: T-BuliDocument4 pagesFukuyama Group - Group Meeting Problems 01/11/2013: T-BuliAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 09/07/2011: O N O Me MeDocument2 pagesFukuyama Group - Group Meeting Problems 09/07/2011: O N O Me MeAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 07/17/2011: Low Yield Under Basic ConditionDocument3 pagesFukuyama Group - Group Meeting Problems 07/17/2011: Low Yield Under Basic ConditionAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 10/06/2012: J. Rodriguez Et Al., Org. Lett., 12, 4212 (2010)Document3 pagesFukuyama Group - Group Meeting Problems 10/06/2012: J. Rodriguez Et Al., Org. Lett., 12, 4212 (2010)Anonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 2/15/2012: Et Si Siet O D Thf-Hmpa - 78 °C 80% Et Si Siet Oh DDocument1 pageFukuyama Group - Group Meeting Problems 2/15/2012: Et Si Siet O D Thf-Hmpa - 78 °C 80% Et Si Siet Oh DAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 11/10/2012: PH BF KDocument3 pagesFukuyama Group - Group Meeting Problems 11/10/2012: PH BF KAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 12/12/2012: CO Me NDocument2 pagesFukuyama Group - Group Meeting Problems 12/12/2012: CO Me NAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 10/01/2011: N-Buli (2 Eq), - 20 °CDocument2 pagesFukuyama Group - Group Meeting Problems 10/01/2011: N-Buli (2 Eq), - 20 °CAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 08/01/2012: Otes MeDocument3 pagesFukuyama Group - Group Meeting Problems 08/01/2012: Otes MeAnonymous rhUNqC1s0No ratings yet
- A 1204Document7 pagesA 1204Anonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 06/02/2010: O P N O Ome OmeDocument2 pagesFukuyama Group - Group Meeting Problems 06/02/2010: O P N O Ome OmeAnonymous rhUNqC1s0No ratings yet
- Fukuyama Group - Group Meeting Problems 12/11/2010: T-BuomeDocument1 pageFukuyama Group - Group Meeting Problems 12/11/2010: T-BuomeAnonymous rhUNqC1s0No ratings yet