Professional Documents
Culture Documents
OCHEM Problem Set
OCHEM Problem Set
Uploaded by
Mandeep SidhuCopyright:
Available Formats
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- ch5 Stereo1 PDFDocument6 pagesch5 Stereo1 PDFyeateshwarriorNo ratings yet
- Organic Chemistry 231 Final ExamDocument19 pagesOrganic Chemistry 231 Final ExamAlex Rose100% (2)
- Final Exam Review Fall 2009 AnswersDocument14 pagesFinal Exam Review Fall 2009 AnswersCharisma SubaNo ratings yet
- Tutorial 2Document5 pagesTutorial 2Joshua LopezNo ratings yet
- Stereochemistry Practce PDFDocument6 pagesStereochemistry Practce PDFFerminNo ratings yet
- Tutorial 2. Stereochemistry PDFDocument5 pagesTutorial 2. Stereochemistry PDFMatthew PokNo ratings yet
- Action Center 1 Constitutional IsomersDocument1 pageAction Center 1 Constitutional IsomersooitzandyooNo ratings yet
- Exam 1 2022 KEYDocument5 pagesExam 1 2022 KEYcody smithNo ratings yet
- Worksheet For Organic SectionDocument17 pagesWorksheet For Organic SectionPramudith Liyanage100% (2)
- Organic Chemistry Structure and BondingDocument13 pagesOrganic Chemistry Structure and BondingHossNo ratings yet
- O-Chem II Exam 2Document17 pagesO-Chem II Exam 2NayeNo ratings yet
- CHM 1321 Assignment 3 - : AnswersDocument5 pagesCHM 1321 Assignment 3 - : AnswersSara YuenNo ratings yet
- CHM133 PRELIM EXAM Part2Document7 pagesCHM133 PRELIM EXAM Part2Rohaisa FaisalNo ratings yet
- Organic Chemistry 3A Additional Problems Final Exam Part 1Document7 pagesOrganic Chemistry 3A Additional Problems Final Exam Part 1John SmithNo ratings yet
- Test 4Document24 pagesTest 4TomNo ratings yet
- Stereochemistry TutorialDocument8 pagesStereochemistry TutorialfezilephathiswaNo ratings yet
- TestbankDocument6 pagesTestbankRen H. RoxasNo ratings yet
- Practice Questions For Ch. 5Document6 pagesPractice Questions For Ch. 5Abeer IbrahiemNo ratings yet
- Practice Questions For Ch. 5Document6 pagesPractice Questions For Ch. 5Abeer IbrahiemNo ratings yet
- 235 Practice Exam 1Document11 pages235 Practice Exam 1bamforNo ratings yet
- Problem Set 1 PDFDocument2 pagesProblem Set 1 PDFLouisNo ratings yet
- CH1O3 Questions PDFDocument52 pagesCH1O3 Questions PDFPrince T MashandaNo ratings yet
- Ch1 2 3 ExercisesDocument11 pagesCh1 2 3 ExercisesMancini100% (1)
- Chap 1 AssignDocument7 pagesChap 1 AssignJianqi NiHao ChenNo ratings yet
- Organic Chemistry Exam 1 (Practice) Chem 237Document3 pagesOrganic Chemistry Exam 1 (Practice) Chem 237Ngoc Minh NgoNo ratings yet
- Chem 31 Probset First ExamDocument2 pagesChem 31 Probset First ExamNat DabuétNo ratings yet
- Tutorial 1 Chapter 1: Introduction To Organic Chemistry: C C H CN H HDocument4 pagesTutorial 1 Chapter 1: Introduction To Organic Chemistry: C C H CN H HRAIN9393No ratings yet
- Mcqs Chemistry Sample PracticeDocument3 pagesMcqs Chemistry Sample PracticeWajid Ali0% (1)
- Organic Chemistry Questions 3Document12 pagesOrganic Chemistry Questions 3Ram KrishnaNo ratings yet
- Sample Paper Gr11Document3 pagesSample Paper Gr11Enoca AJNo ratings yet
- 3.1.1 Naming Practice QuestionsDocument33 pages3.1.1 Naming Practice QuestionsAyaan RaufNo ratings yet
- 1 AllDocument18 pages1 AllMarcos ViníciusNo ratings yet
- Exam 1 Review 1 KOTDocument47 pagesExam 1 Review 1 KOTNoranisza MahmudNo ratings yet
- Assignment 2 BLC F20Document3 pagesAssignment 2 BLC F20Rémi MartineauNo ratings yet
- Chem Exam 4Document30 pagesChem Exam 4TomNo ratings yet
- 11 GocDocument2 pages11 GocHarsh SinghNo ratings yet
- Introduction To Organic Chemistry Ii CHEM 224: Answer KeyDocument8 pagesIntroduction To Organic Chemistry Ii CHEM 224: Answer Keygautamtajesh1983No ratings yet
- CHM 1321 Assignment 1 Answers: CN H H H H HDocument10 pagesCHM 1321 Assignment 1 Answers: CN H H H H HSara YuenNo ratings yet
- Organic Chemistry Additional Problems Final Exam Part2Document6 pagesOrganic Chemistry Additional Problems Final Exam Part2John SmithNo ratings yet
- Tutorial 1Document5 pagesTutorial 1leftphoneforeverNo ratings yet
- Chem 112A F11 Homework 3Document6 pagesChem 112A F11 Homework 3Shyam BhaktaNo ratings yet
- OCW Exam 1Document10 pagesOCW Exam 1iliketospam123No ratings yet
- Chem 343 Exam 1 KeyDocument6 pagesChem 343 Exam 1 Key蔡蕲No ratings yet
- Objectives: UNIT 3. HydrocarbonsDocument52 pagesObjectives: UNIT 3. HydrocarbonsMarcelaNo ratings yet
- Ass 4 Bonding IDocument5 pagesAss 4 Bonding IYuvraj UbovejaNo ratings yet
- Exam 1 BDocument6 pagesExam 1 BTouqueer AhmedNo ratings yet
- Practice Problems For Chapter 3: CH CH CHCH CH CH C (CH) CH CHDocument4 pagesPractice Problems For Chapter 3: CH CH CHCH CH CH C (CH) CH CHankitguhesmailboxNo ratings yet
- CHM 2210 Practice Ex I If 12Document10 pagesCHM 2210 Practice Ex I If 12Shaima MossamatNo ratings yet
- Test 2 ReviewDocument7 pagesTest 2 Reviewserenity1290100% (1)
- Additional Problems Final Exam Part 2 AnswersDocument10 pagesAdditional Problems Final Exam Part 2 AnswersJohn SmithNo ratings yet
- Chemistry Unit ReviewDocument4 pagesChemistry Unit ReviewnishilgeorgeNo ratings yet
- Chem 112A Homework 1Document4 pagesChem 112A Homework 1Shyam BhaktaNo ratings yet
- Latihan Soal - 1Document3 pagesLatihan Soal - 1Jonny PNo ratings yet
- Test - 2 - Solutions and FeedbackDocument7 pagesTest - 2 - Solutions and FeedbackNondumiso MavundlaNo ratings yet
- Infrared Spectroscopy of Triatomics for Space ObservationFrom EverandInfrared Spectroscopy of Triatomics for Space ObservationNo ratings yet
- Main Group Metal Coordination Polymers: Structures and NanostructuresFrom EverandMain Group Metal Coordination Polymers: Structures and NanostructuresNo ratings yet
OCHEM Problem Set
OCHEM Problem Set
Uploaded by
Mandeep SidhuOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
OCHEM Problem Set
OCHEM Problem Set
Uploaded by
Mandeep SidhuCopyright:
Available Formats
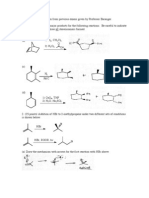
Problem Set #1
Chem 260
1.
HN
C
O
HN
O
N
What is the hybridization state of the following
atoms?
Name the circled functional groups.
2. Consider the conformations about the C4-C3 bond of the molecule shown below (view as
indicated by the arrow). Complete the Newman projections below using only staggered and
eclipsed conformations to indicate the order of stability of conformations about this bond (starting
with the most stable one and ending with the least stable one).
5
3
view
H3 C
H H3 C
CH(CH3)2
most stable
H3 C
H H3C
CH(CH3)2
2nd
H H3 C
CH(CH3)2
4th
CH(CH3)2
3rd
H H3C
CH(CH3)2
5th
CH(CH3)2
least stable
3. Name the following compounds using IUPAC nomenclature.
a)
b)
c)
OH
d)
e)
CH3
Cl
4. Draw all constitutional isomers with the formula C4H10O.
5. Consider the molecule drawn below.
H2N
CO2H
N
HO
Draw the form of this compound that would be obtained if it was dissolved in a non-aqueous
solvent and treated with:
a) one equivalent of the very strong base CH3- Li+
b) two equivalents of the very strong base CH3- Li+
6. The alcohol shown below has a much lower pKa than a normal alcohol.
HO
NO2
a) Draw the anion formed upon deprotonation of this alcohol showing all lone pairs and formal
charges.
b) Draw all relevant resonance structures of the anion that help explain why the pKa of the
alcohol is low.
7. a) Give a line drawing for the molecule ozone (O3) showing all lone pairs and formal charges.
b) Is ozone a linear or bent molecule? Explain.
c) Draw two resonance structures that contribute most to the overall picture of ozone.
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- ch5 Stereo1 PDFDocument6 pagesch5 Stereo1 PDFyeateshwarriorNo ratings yet
- Organic Chemistry 231 Final ExamDocument19 pagesOrganic Chemistry 231 Final ExamAlex Rose100% (2)
- Final Exam Review Fall 2009 AnswersDocument14 pagesFinal Exam Review Fall 2009 AnswersCharisma SubaNo ratings yet
- Tutorial 2Document5 pagesTutorial 2Joshua LopezNo ratings yet
- Stereochemistry Practce PDFDocument6 pagesStereochemistry Practce PDFFerminNo ratings yet
- Tutorial 2. Stereochemistry PDFDocument5 pagesTutorial 2. Stereochemistry PDFMatthew PokNo ratings yet
- Action Center 1 Constitutional IsomersDocument1 pageAction Center 1 Constitutional IsomersooitzandyooNo ratings yet
- Exam 1 2022 KEYDocument5 pagesExam 1 2022 KEYcody smithNo ratings yet
- Worksheet For Organic SectionDocument17 pagesWorksheet For Organic SectionPramudith Liyanage100% (2)
- Organic Chemistry Structure and BondingDocument13 pagesOrganic Chemistry Structure and BondingHossNo ratings yet
- O-Chem II Exam 2Document17 pagesO-Chem II Exam 2NayeNo ratings yet
- CHM 1321 Assignment 3 - : AnswersDocument5 pagesCHM 1321 Assignment 3 - : AnswersSara YuenNo ratings yet
- CHM133 PRELIM EXAM Part2Document7 pagesCHM133 PRELIM EXAM Part2Rohaisa FaisalNo ratings yet
- Organic Chemistry 3A Additional Problems Final Exam Part 1Document7 pagesOrganic Chemistry 3A Additional Problems Final Exam Part 1John SmithNo ratings yet
- Test 4Document24 pagesTest 4TomNo ratings yet
- Stereochemistry TutorialDocument8 pagesStereochemistry TutorialfezilephathiswaNo ratings yet
- TestbankDocument6 pagesTestbankRen H. RoxasNo ratings yet
- Practice Questions For Ch. 5Document6 pagesPractice Questions For Ch. 5Abeer IbrahiemNo ratings yet
- Practice Questions For Ch. 5Document6 pagesPractice Questions For Ch. 5Abeer IbrahiemNo ratings yet
- 235 Practice Exam 1Document11 pages235 Practice Exam 1bamforNo ratings yet
- Problem Set 1 PDFDocument2 pagesProblem Set 1 PDFLouisNo ratings yet
- CH1O3 Questions PDFDocument52 pagesCH1O3 Questions PDFPrince T MashandaNo ratings yet
- Ch1 2 3 ExercisesDocument11 pagesCh1 2 3 ExercisesMancini100% (1)
- Chap 1 AssignDocument7 pagesChap 1 AssignJianqi NiHao ChenNo ratings yet
- Organic Chemistry Exam 1 (Practice) Chem 237Document3 pagesOrganic Chemistry Exam 1 (Practice) Chem 237Ngoc Minh NgoNo ratings yet
- Chem 31 Probset First ExamDocument2 pagesChem 31 Probset First ExamNat DabuétNo ratings yet
- Tutorial 1 Chapter 1: Introduction To Organic Chemistry: C C H CN H HDocument4 pagesTutorial 1 Chapter 1: Introduction To Organic Chemistry: C C H CN H HRAIN9393No ratings yet
- Mcqs Chemistry Sample PracticeDocument3 pagesMcqs Chemistry Sample PracticeWajid Ali0% (1)
- Organic Chemistry Questions 3Document12 pagesOrganic Chemistry Questions 3Ram KrishnaNo ratings yet
- Sample Paper Gr11Document3 pagesSample Paper Gr11Enoca AJNo ratings yet
- 3.1.1 Naming Practice QuestionsDocument33 pages3.1.1 Naming Practice QuestionsAyaan RaufNo ratings yet
- 1 AllDocument18 pages1 AllMarcos ViníciusNo ratings yet
- Exam 1 Review 1 KOTDocument47 pagesExam 1 Review 1 KOTNoranisza MahmudNo ratings yet
- Assignment 2 BLC F20Document3 pagesAssignment 2 BLC F20Rémi MartineauNo ratings yet
- Chem Exam 4Document30 pagesChem Exam 4TomNo ratings yet
- 11 GocDocument2 pages11 GocHarsh SinghNo ratings yet
- Introduction To Organic Chemistry Ii CHEM 224: Answer KeyDocument8 pagesIntroduction To Organic Chemistry Ii CHEM 224: Answer Keygautamtajesh1983No ratings yet
- CHM 1321 Assignment 1 Answers: CN H H H H HDocument10 pagesCHM 1321 Assignment 1 Answers: CN H H H H HSara YuenNo ratings yet
- Organic Chemistry Additional Problems Final Exam Part2Document6 pagesOrganic Chemistry Additional Problems Final Exam Part2John SmithNo ratings yet
- Tutorial 1Document5 pagesTutorial 1leftphoneforeverNo ratings yet
- Chem 112A F11 Homework 3Document6 pagesChem 112A F11 Homework 3Shyam BhaktaNo ratings yet
- OCW Exam 1Document10 pagesOCW Exam 1iliketospam123No ratings yet
- Chem 343 Exam 1 KeyDocument6 pagesChem 343 Exam 1 Key蔡蕲No ratings yet
- Objectives: UNIT 3. HydrocarbonsDocument52 pagesObjectives: UNIT 3. HydrocarbonsMarcelaNo ratings yet
- Ass 4 Bonding IDocument5 pagesAss 4 Bonding IYuvraj UbovejaNo ratings yet
- Exam 1 BDocument6 pagesExam 1 BTouqueer AhmedNo ratings yet
- Practice Problems For Chapter 3: CH CH CHCH CH CH C (CH) CH CHDocument4 pagesPractice Problems For Chapter 3: CH CH CHCH CH CH C (CH) CH CHankitguhesmailboxNo ratings yet
- CHM 2210 Practice Ex I If 12Document10 pagesCHM 2210 Practice Ex I If 12Shaima MossamatNo ratings yet
- Test 2 ReviewDocument7 pagesTest 2 Reviewserenity1290100% (1)
- Additional Problems Final Exam Part 2 AnswersDocument10 pagesAdditional Problems Final Exam Part 2 AnswersJohn SmithNo ratings yet
- Chemistry Unit ReviewDocument4 pagesChemistry Unit ReviewnishilgeorgeNo ratings yet
- Chem 112A Homework 1Document4 pagesChem 112A Homework 1Shyam BhaktaNo ratings yet
- Latihan Soal - 1Document3 pagesLatihan Soal - 1Jonny PNo ratings yet
- Test - 2 - Solutions and FeedbackDocument7 pagesTest - 2 - Solutions and FeedbackNondumiso MavundlaNo ratings yet
- Infrared Spectroscopy of Triatomics for Space ObservationFrom EverandInfrared Spectroscopy of Triatomics for Space ObservationNo ratings yet
- Main Group Metal Coordination Polymers: Structures and NanostructuresFrom EverandMain Group Metal Coordination Polymers: Structures and NanostructuresNo ratings yet