Professional Documents
Culture Documents
Constitution of Alloys and Phase Diagrams: 1.2 - Eutectoid, Eutectic, Peritectic, and Peritectroid Reactions
Constitution of Alloys and Phase Diagrams: 1.2 - Eutectoid, Eutectic, Peritectic, and Peritectroid Reactions
Uploaded by
Kavin Balasubramaniam0 ratings0% found this document useful (0 votes)
26 views5 pagesThis document discusses phase diagrams and reactions in iron-carbon alloys. It describes the different phases present in hypoeutectoid, eutectoid, and hypereutectoid steels, as well as cast irons, based on their carbon content. It also outlines the eutectoid reaction where austenite transforms directly to a layered structure called pearlite consisting of ferrite and cementite layers.
Original Description:
Iron carbide diagram
Original Title
1.2
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document discusses phase diagrams and reactions in iron-carbon alloys. It describes the different phases present in hypoeutectoid, eutectoid, and hypereutectoid steels, as well as cast irons, based on their carbon content. It also outlines the eutectoid reaction where austenite transforms directly to a layered structure called pearlite consisting of ferrite and cementite layers.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
26 views5 pagesConstitution of Alloys and Phase Diagrams: 1.2 - Eutectoid, Eutectic, Peritectic, and Peritectroid Reactions
Constitution of Alloys and Phase Diagrams: 1.2 - Eutectoid, Eutectic, Peritectic, and Peritectroid Reactions
Uploaded by
Kavin BalasubramaniamThis document discusses phase diagrams and reactions in iron-carbon alloys. It describes the different phases present in hypoeutectoid, eutectoid, and hypereutectoid steels, as well as cast irons, based on their carbon content. It also outlines the eutectoid reaction where austenite transforms directly to a layered structure called pearlite consisting of ferrite and cementite layers.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 5
UNIT-I
CONSTITUTION OF ALLOYS AND PHASE DIAGRAMS
1.2 - Eutectoid, eutectic, peritectic, and peritectroid reactions
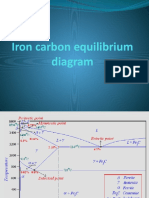
Phase compositions of the iron-carbon alloys at room temperature
Hypoeutectoid steels (carbon content from 0 to 0.83%) consist of
primary (proeutectoid) ferrite (according to the curve A3) and pearlite.
Eutectoid steel (carbon content 0.83%) entirely consists of pearlite.
Hypereutectoid steels (carbon content from 0.83 to 2.06%) consist of
primary (proeutectoid)cementite (according to the curve ACM) and
pearlite.
Cast irons (carbon content from 2.06% to 4.3%) consist of proeutectoid
cementite C2 ejected from austenite according to the curve ACM ,
pearlite and transformed ledeburite (ledeburite in which austenite
transformed to pearlite).
At 4.3% carbon composition, on cooling Liquid phase is converted in to two
solids hence forming Eutectic reaction.
L + Fe3C
Eutectoid: 0.76 wt%C, 727 C
(0.76 wt% C) (0.022 wt% C) + Fe3C
Shown below is the steel part of the iron carbon diagram containing up to
2% Carbon. At the eutectoid point 0.83% Carbon, Austenite which is in a
solid solution changes directly into a solid known as Pearlite which is
a layered structure consisting of layers of Ferrite and Cementite
You might also like
- Chapt 09Document34 pagesChapt 09Jesse McClure75% (8)
- Iron Carbon Phase DiagramDocument7 pagesIron Carbon Phase Diagrampratap biswasNo ratings yet
- Iron Carbon Fe-C Equilibrium Diagram - An Iron Carbon EquilibriumDocument2 pagesIron Carbon Fe-C Equilibrium Diagram - An Iron Carbon Equilibriumروشان فاطمة روشانNo ratings yet
- Iron Carbon SystemDocument14 pagesIron Carbon SystemPraicy Ann josephNo ratings yet
- Iron Carbon Phase DiagramDocument3 pagesIron Carbon Phase DiagramrabikmNo ratings yet
- 9 Engineering AlloysDocument17 pages9 Engineering AlloysdavidtomyNo ratings yet
- Iron Carbon Note 1 2023Document23 pagesIron Carbon Note 1 2023gerrard samuelNo ratings yet
- Iron-Carbon Phase Diagram (SubsTech)Document2 pagesIron-Carbon Phase Diagram (SubsTech)Aboo BackerNo ratings yet
- Iron-Iron Carbide DiagramDocument10 pagesIron-Iron Carbide DiagrammusabNo ratings yet
- SteelDocument4 pagesSteelgovimanoNo ratings yet
- Sudipta Nath: Materials EngineeringDocument19 pagesSudipta Nath: Materials EngineeringSudipta NathNo ratings yet
- Diagram FasaDocument6 pagesDiagram Fasaolid_zoneNo ratings yet
- Iron Carbon DiagramDocument10 pagesIron Carbon DiagramsivakumarNo ratings yet
- Capili Jefferson 10Document5 pagesCapili Jefferson 10Christian Al EncarnacionNo ratings yet
- Iron Carbon Part1 PDFDocument33 pagesIron Carbon Part1 PDFErick HoganNo ratings yet
- Alloys - Steel-Hypo and Hyper EutectoidDocument46 pagesAlloys - Steel-Hypo and Hyper EutectoidLavanya PriyaNo ratings yet
- Tutorial Questions 3-ContinuedDocument2 pagesTutorial Questions 3-ContinuedGRAHAM KUNDAI DENGEZANo ratings yet
- Ch-27.5 Iron Carbon Equilibrium DiagramDocument52 pagesCh-27.5 Iron Carbon Equilibrium DiagramManojNo ratings yet
- Ch-27.3 Iron Carbon Equilibrium DiagramDocument58 pagesCh-27.3 Iron Carbon Equilibrium DiagramasjfgauojfgfNo ratings yet
- 3 Iron Carbon DiaDocument21 pages3 Iron Carbon DiaChhavi SharmaNo ratings yet
- Unit 3Document113 pagesUnit 3Abhishek ChavanNo ratings yet
- Iron-Carbon Phase Diagram Explained BrieflyDocument4 pagesIron-Carbon Phase Diagram Explained BrieflyZicoNo ratings yet
- IIC DiagramDocument57 pagesIIC DiagramAbhishek ChavanNo ratings yet
- Lec 7 Fe C DiagramDocument45 pagesLec 7 Fe C DiagramAdnan MehmoodNo ratings yet
- Iron Carbon Equilibrium DiagramDocument52 pagesIron Carbon Equilibrium DiagramSohan Lal100% (2)
- Eng Mat Chapter 4Document126 pagesEng Mat Chapter 4VC Chua Yee LeongNo ratings yet
- Assignment TwoDocument3 pagesAssignment TwoAnthony MubangaNo ratings yet
- Yan Sample Exam II 4016214112Document6 pagesYan Sample Exam II 4016214112christopher_spring_3No ratings yet
- Alloys & Their Phase Diagrams Alloys & Their Phase DiagramsDocument52 pagesAlloys & Their Phase Diagrams Alloys & Their Phase DiagramselgawadhaNo ratings yet
- The Iron-Carbon Phase Diagram: Prof. H. K. Khaira Professor in MSME Deptt. MANIT, BhopalDocument38 pagesThe Iron-Carbon Phase Diagram: Prof. H. K. Khaira Professor in MSME Deptt. MANIT, BhopalYogesh KumbharNo ratings yet
- Ch-27.5 Iron Carbon Equilibrium DiagramDocument53 pagesCh-27.5 Iron Carbon Equilibrium DiagramSmruti Ranjan PattanayakNo ratings yet
- The IronCarbide DiagramDocument11 pagesThe IronCarbide DiagramshajjikhalidNo ratings yet
- Ironiron CarbideequilibriumphasediagramDocument39 pagesIroniron CarbideequilibriumphasediagramSheikh UMARNo ratings yet
- MSM GTU Study Material E-Notes Unit-5 23112020052908AMDocument14 pagesMSM GTU Study Material E-Notes Unit-5 23112020052908AMVijayNo ratings yet
- The iron-iron carbide (Fe-Fe C) phase diagram: α-ferrite austeniteDocument7 pagesThe iron-iron carbide (Fe-Fe C) phase diagram: α-ferrite austenitepgp655484No ratings yet
- Iron-Iron Carbide Phase Diagram ExampleDocument4 pagesIron-Iron Carbide Phase Diagram ExampleWanda Andreas WidyatmokoNo ratings yet
- Iron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Document33 pagesIron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Mahmoud RefaatNo ratings yet
- Phase Diagram of Fe-Fe3CDocument25 pagesPhase Diagram of Fe-Fe3CIram MustaviNo ratings yet
- Iron-Carbon Phase DiagramDocument30 pagesIron-Carbon Phase Diagramjunaid hassanNo ratings yet
- Iron-Iron Carbide Phase Diagram ExampleDocument3 pagesIron-Iron Carbide Phase Diagram ExampleBenjamin Enmanuel Mango DNo ratings yet
- Study of Iron-Iron Carbide and Isothermal TransformationDocument14 pagesStudy of Iron-Iron Carbide and Isothermal TransformationAhmed JishanNo ratings yet
- Iron Carbon Equillibrium Diagram GandhidhamDocument22 pagesIron Carbon Equillibrium Diagram Gandhidhamcal2_uniNo ratings yet
- Unit-2 Notes (Material Science)Document24 pagesUnit-2 Notes (Material Science)AMAN SINGHNo ratings yet
- EMM LectureDocument38 pagesEMM Lecturelatendra kumar srivastavNo ratings yet
- Iron - Carbon Phase Diagram: Sandeep Nair CB - EN.P2MFG15018Document30 pagesIron - Carbon Phase Diagram: Sandeep Nair CB - EN.P2MFG15018prasenjitsayantan100% (1)
- Iron Carbon DiagramDocument8 pagesIron Carbon Diagramashok pradhanNo ratings yet
- Iron Ironcarbidediagram 161008052257Document41 pagesIron Ironcarbidediagram 161008052257Siddharth Sharma100% (1)
- Hypoeutectiod Steel Alloys - SteelDocument52 pagesHypoeutectiod Steel Alloys - SteelnotsofarNo ratings yet
- Iron Carbon DiagramDocument9 pagesIron Carbon DiagramNagamuthu PandianNo ratings yet
- Introduction To Fe-C Equilibrium Phase Diagram: Chia-Chang ShihDocument42 pagesIntroduction To Fe-C Equilibrium Phase Diagram: Chia-Chang ShihTuấnPhạmNo ratings yet
- Iron-Carbon DiagramDocument3 pagesIron-Carbon DiagramnaniNo ratings yet
- Industrial Materials (B)Document15 pagesIndustrial Materials (B)Muhammad Bilal SahiNo ratings yet
- Metastable Iron-Carbon (Fe-C) Phase DiagramDocument3 pagesMetastable Iron-Carbon (Fe-C) Phase DiagramupenderNo ratings yet
- Section 2: The Microstructural Nature of Carbon SteelsDocument4 pagesSection 2: The Microstructural Nature of Carbon Steelsamitkharb111195No ratings yet
- Endohedral Metallofullerenes: Fullerenes with Metal InsideFrom EverandEndohedral Metallofullerenes: Fullerenes with Metal InsideNo ratings yet
- Vibrational Spectra of Organometallics: Theoretical and Experimental DataFrom EverandVibrational Spectra of Organometallics: Theoretical and Experimental DataNo ratings yet
- Advanced Battery MaterialsFrom EverandAdvanced Battery MaterialsChunwen SunNo ratings yet
- Metallabenzenes: An Expert ViewFrom EverandMetallabenzenes: An Expert ViewL. James WrightNo ratings yet
- Fundamentals of Electrocatalyst Materials and Interfacial Characterization: Energy Producing Devices and Environmental ProtectionFrom EverandFundamentals of Electrocatalyst Materials and Interfacial Characterization: Energy Producing Devices and Environmental ProtectionNo ratings yet
- Assignment: On Finding TMT Rod Composition Using Utm ResultsDocument7 pagesAssignment: On Finding TMT Rod Composition Using Utm ResultsKavin BalasubramaniamNo ratings yet
- V BeltDocument15 pagesV BeltKavin BalasubramaniamNo ratings yet
- Mechanical MCQDocument2 pagesMechanical MCQKavin BalasubramaniamNo ratings yet
- Gear Ratios TorqueDocument12 pagesGear Ratios TorqueKavin BalasubramaniamNo ratings yet
- Ex - No: Date:: Kgisl Institute of Technology - KiteDocument3 pagesEx - No: Date:: Kgisl Institute of Technology - KiteKavin BalasubramaniamNo ratings yet
- RC Car Racing: Rules and RegulationDocument1 pageRC Car Racing: Rules and RegulationKavin BalasubramaniamNo ratings yet