Professional Documents
Culture Documents
Chemical Science 8 FPD Periodic Table
Chemical Science 8 FPD Periodic Table
Uploaded by
api-280679159Copyright:
Available Formats
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5835)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Hancock (2010-03) Momentum - A Contrarian Case For Following The HerdDocument13 pagesHancock (2010-03) Momentum - A Contrarian Case For Following The HerddrummondjacobNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- 60 Minutes-60 Questions: Mathematics TestDocument14 pages60 Minutes-60 Questions: Mathematics TestJihyun YeonNo ratings yet
- Project PlanDocument2 pagesProject Planapi-280679159No ratings yet
- Chemical Science 8 FPD FinalDocument2 pagesChemical Science 8 FPD Finalapi-280679159No ratings yet
- Ict Forward Planning DocumentDocument2 pagesIct Forward Planning Documentapi-280679159No ratings yet
- Ict Lesson 4 Lesson PlanDocument4 pagesIct Lesson 4 Lesson Planapi-280679159No ratings yet
- Free Mastic G 316Document5 pagesFree Mastic G 316Kelly RobertsNo ratings yet
- Smart World 10 - Unit 2 TestDocument6 pagesSmart World 10 - Unit 2 TestSally VuNo ratings yet
- Aflac Claim FormDocument7 pagesAflac Claim FormThomas Barrett100% (2)
- Philosophies and Theories of EducationDocument26 pagesPhilosophies and Theories of Educationcynthiaaa sNo ratings yet
- Gordon 2009 CatalogDocument68 pagesGordon 2009 Catalogpolanck100% (3)
- Unfinished Pow UTOLDocument251 pagesUnfinished Pow UTOLresty tacataNo ratings yet
- DATA SUMMARIZATION - PrintDocument28 pagesDATA SUMMARIZATION - PrintThành Cao ĐứcNo ratings yet
- 98 EntrepreneurshipDocument11 pages98 Entrepreneurshipdolly gorlakorakanaNo ratings yet
- Silo - Tips - Pier 1 Bored Pile Details PDFDocument11 pagesSilo - Tips - Pier 1 Bored Pile Details PDFJanno NietoNo ratings yet
- Non-Local Consciousness and The Anthropo PDFDocument4 pagesNon-Local Consciousness and The Anthropo PDFtoffaloniNo ratings yet
- GU Balance Routine Testing enDocument12 pagesGU Balance Routine Testing enasalazarsNo ratings yet
- Respiratory DiseaseDocument27 pagesRespiratory Diseaseعبدالسلام الأسمرNo ratings yet
- Guidelines For Education and TrainingDocument9 pagesGuidelines For Education and Trainingsunrise755No ratings yet
- Development of Stainless Steel Sheets For Cylinder Head GasketDocument7 pagesDevelopment of Stainless Steel Sheets For Cylinder Head Gasketstefan.vince536No ratings yet
- List of Approved Vendors QAM 25.04.2017 FinalDocument59 pagesList of Approved Vendors QAM 25.04.2017 FinalMayank Singhania100% (1)
- Type 9, 12, 16, 20, 24 & 36 Brake Chamber (SD-02-1302)Document4 pagesType 9, 12, 16, 20, 24 & 36 Brake Chamber (SD-02-1302)Angel Dlsg100% (1)
- QMLN 01 Schrödinger EquationDocument10 pagesQMLN 01 Schrödinger EquationAnum Hosen ShawonNo ratings yet
- Ice Melting Methods For Overhead LinesDocument28 pagesIce Melting Methods For Overhead LinesKirana Shree100% (1)
- RamaDocument48 pagesRamaSebastián Diez CáceresNo ratings yet
- GGN DD 033 - D0 PDFDocument250 pagesGGN DD 033 - D0 PDFAkshit KhuranaNo ratings yet
- Hitler and GermanentumDocument17 pagesHitler and Germanentumlord azraelNo ratings yet
- 1,2 3Document10 pages1,2 3Nicole A. De GuzmanNo ratings yet
- Final MINUTES For 63rd ESC Meeting 17.03.2020Document59 pagesFinal MINUTES For 63rd ESC Meeting 17.03.2020Girish YadavNo ratings yet
- Fairfield Atlas Limited Shinoli Metallurgical Technical Delivery Specification TDS No: TDS/ P 0110790 Rev No: 02 Page No: 1 of 3Document4 pagesFairfield Atlas Limited Shinoli Metallurgical Technical Delivery Specification TDS No: TDS/ P 0110790 Rev No: 02 Page No: 1 of 3Andras StegerNo ratings yet
- For Review OAPDocument144 pagesFor Review OAPKim John Rull NateNo ratings yet
- Teacher Quality StandardsDocument9 pagesTeacher Quality StandardsPaulina WilkersonNo ratings yet
- Design and Manufacturing of A Prototype Multipurpose Robot Capable of Military ActionDocument6 pagesDesign and Manufacturing of A Prototype Multipurpose Robot Capable of Military ActionInternational Journal of Application or Innovation in Engineering & ManagementNo ratings yet
Chemical Science 8 FPD Periodic Table
Chemical Science 8 FPD Periodic Table
Uploaded by
api-280679159Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemical Science 8 FPD Periodic Table
Chemical Science 8 FPD Periodic Table
Uploaded by
api-280679159Copyright:
Available Formats
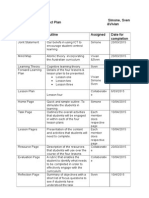
FORWARD PLANNING DOCUMENT
TERM/WEEKS: Term
Weeks 3-5
YEAR LEVEL: 8
LEARNING AREA/TOPIC: Chemical Science
AUSTRALIAN CURRICULUM
General Capabilities:
Literacy
Numeracy
ICT
Cross-curriculum priorities:
Aboriginal and Torres Strait Islander histories and
Cultures
WEEK
/
LESS
ON
AUSTRALIAN
CURRICULUM
LINKS
Science
Underst
anding
Science
as a
Human
Endeavo
ur
ACSSU152
ACSHE134
Critical and creative
thinking
Ethical
Behaviour
Asia and Australias engagement with Asia
Personal and social
Competence
Intercultural
Understanding
Sustainability
SPECIFIC
LESSON
OBJECTIVE
ASSESSMENT
(what & how)
TEACHING & LEARNING
EXPERIENCES
(include learner diversity)
KEY
QUESTIONS
Students are able to:
1. State that elements are
substances with only one
type of atom.
2. Identify and describe and
locate elements on the
periodic table.
3. Understand how
Mendeleevs contribution
to chemistry deeply
impacted our knowledge of
the elements and their
properties.
Formative
Class discussions,
answers provided and
questions asked by
students
KWL Charts
Student interaction with
the website.
Remedial: Pair these
students with stronger
students.
Extension: Students
can teach weaker
students why we have
periodic table and how
to find elements.
Elements and the Periodic Table
Intro: Introduce the concept of elements being the simplest substance and
that they are purposefully arranged on Mendeleevs Periodic Table.
Body:
1. Give students a KWL chart and ask them to write what they know about
the topic in K column. Discuss what they know with class.
2. Show YouTube video. Ask the class to Think-Pair-Share, then fill in the W
column with questions about what they want to know. Discuss questions
with class.
3. Explain and reiterate with examples what students heard in the video by
referring to a projection of the periodic table.
4. Ask students to work individually and access the Human touch of
chemistry website. Students must select any element of their choice from
the list provided on the white board and using the interactive periodic table
state - what the element is? What is it used for? How was it discovered
and by who? What is the atomic number?
Conclusion: Students to write what they learned in L column, then hands
up to encourage class discussion on what they learned. Link to next lesson
on properties of elements. Homework: Qs. 1-5 p.254.
Who designed the
periodic table as we
know it today?
Why is the periodic
table the structured
the way it is?
What does the
periodic table tell
us? What doesnt it
tell us?
RESOURCES
Science
Inquiry
Skills
KWL Chart
The human touch of
chemistry
http://humantouchof
chemistry.com/perio
dic-table.htm#
TED-Ed Lesson: The
genius of
Mendeleev's periodic
table
https://www.youtube.c
om/watch?
v=fPnwBITSmgU
Projection of
Periodic table
Pearson Science
year 8 pg 252-254
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5835)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Hancock (2010-03) Momentum - A Contrarian Case For Following The HerdDocument13 pagesHancock (2010-03) Momentum - A Contrarian Case For Following The HerddrummondjacobNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- 60 Minutes-60 Questions: Mathematics TestDocument14 pages60 Minutes-60 Questions: Mathematics TestJihyun YeonNo ratings yet
- Project PlanDocument2 pagesProject Planapi-280679159No ratings yet
- Chemical Science 8 FPD FinalDocument2 pagesChemical Science 8 FPD Finalapi-280679159No ratings yet
- Ict Forward Planning DocumentDocument2 pagesIct Forward Planning Documentapi-280679159No ratings yet
- Ict Lesson 4 Lesson PlanDocument4 pagesIct Lesson 4 Lesson Planapi-280679159No ratings yet
- Free Mastic G 316Document5 pagesFree Mastic G 316Kelly RobertsNo ratings yet
- Smart World 10 - Unit 2 TestDocument6 pagesSmart World 10 - Unit 2 TestSally VuNo ratings yet
- Aflac Claim FormDocument7 pagesAflac Claim FormThomas Barrett100% (2)
- Philosophies and Theories of EducationDocument26 pagesPhilosophies and Theories of Educationcynthiaaa sNo ratings yet
- Gordon 2009 CatalogDocument68 pagesGordon 2009 Catalogpolanck100% (3)
- Unfinished Pow UTOLDocument251 pagesUnfinished Pow UTOLresty tacataNo ratings yet
- DATA SUMMARIZATION - PrintDocument28 pagesDATA SUMMARIZATION - PrintThành Cao ĐứcNo ratings yet
- 98 EntrepreneurshipDocument11 pages98 Entrepreneurshipdolly gorlakorakanaNo ratings yet
- Silo - Tips - Pier 1 Bored Pile Details PDFDocument11 pagesSilo - Tips - Pier 1 Bored Pile Details PDFJanno NietoNo ratings yet
- Non-Local Consciousness and The Anthropo PDFDocument4 pagesNon-Local Consciousness and The Anthropo PDFtoffaloniNo ratings yet
- GU Balance Routine Testing enDocument12 pagesGU Balance Routine Testing enasalazarsNo ratings yet
- Respiratory DiseaseDocument27 pagesRespiratory Diseaseعبدالسلام الأسمرNo ratings yet
- Guidelines For Education and TrainingDocument9 pagesGuidelines For Education and Trainingsunrise755No ratings yet
- Development of Stainless Steel Sheets For Cylinder Head GasketDocument7 pagesDevelopment of Stainless Steel Sheets For Cylinder Head Gasketstefan.vince536No ratings yet
- List of Approved Vendors QAM 25.04.2017 FinalDocument59 pagesList of Approved Vendors QAM 25.04.2017 FinalMayank Singhania100% (1)
- Type 9, 12, 16, 20, 24 & 36 Brake Chamber (SD-02-1302)Document4 pagesType 9, 12, 16, 20, 24 & 36 Brake Chamber (SD-02-1302)Angel Dlsg100% (1)
- QMLN 01 Schrödinger EquationDocument10 pagesQMLN 01 Schrödinger EquationAnum Hosen ShawonNo ratings yet
- Ice Melting Methods For Overhead LinesDocument28 pagesIce Melting Methods For Overhead LinesKirana Shree100% (1)
- RamaDocument48 pagesRamaSebastián Diez CáceresNo ratings yet
- GGN DD 033 - D0 PDFDocument250 pagesGGN DD 033 - D0 PDFAkshit KhuranaNo ratings yet
- Hitler and GermanentumDocument17 pagesHitler and Germanentumlord azraelNo ratings yet
- 1,2 3Document10 pages1,2 3Nicole A. De GuzmanNo ratings yet
- Final MINUTES For 63rd ESC Meeting 17.03.2020Document59 pagesFinal MINUTES For 63rd ESC Meeting 17.03.2020Girish YadavNo ratings yet
- Fairfield Atlas Limited Shinoli Metallurgical Technical Delivery Specification TDS No: TDS/ P 0110790 Rev No: 02 Page No: 1 of 3Document4 pagesFairfield Atlas Limited Shinoli Metallurgical Technical Delivery Specification TDS No: TDS/ P 0110790 Rev No: 02 Page No: 1 of 3Andras StegerNo ratings yet
- For Review OAPDocument144 pagesFor Review OAPKim John Rull NateNo ratings yet
- Teacher Quality StandardsDocument9 pagesTeacher Quality StandardsPaulina WilkersonNo ratings yet
- Design and Manufacturing of A Prototype Multipurpose Robot Capable of Military ActionDocument6 pagesDesign and Manufacturing of A Prototype Multipurpose Robot Capable of Military ActionInternational Journal of Application or Innovation in Engineering & ManagementNo ratings yet