Professional Documents
Culture Documents
Half Life
Half Life
Uploaded by
Misty Vito0 ratings0% found this document useful (0 votes)
83 views8 pagesThis document discusses half-life, which is the time it takes for half of the atoms in a radioactive sample to decay. It provides examples of half-lives for various radioisotopes ranging from 5 years to 4.5 billion years. Uranium-238 has a very long half-life of 4.5 billion years and is used for geological dating. The oldest rocks on Earth are around 3.7 billion years old based on uranium-238 dating. Several examples are given to illustrate how to calculate the amount of radioactive material remaining after a given number of half-lives. The rate of radioactive decay, characterized by half-life, remains constant regardless of external conditions like pressure and temperature.
Original Description:
Physics
Original Title
HALF-LIFE
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document discusses half-life, which is the time it takes for half of the atoms in a radioactive sample to decay. It provides examples of half-lives for various radioisotopes ranging from 5 years to 4.5 billion years. Uranium-238 has a very long half-life of 4.5 billion years and is used for geological dating. The oldest rocks on Earth are around 3.7 billion years old based on uranium-238 dating. Several examples are given to illustrate how to calculate the amount of radioactive material remaining after a given number of half-lives. The rate of radioactive decay, characterized by half-life, remains constant regardless of external conditions like pressure and temperature.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
83 views8 pagesHalf Life
Half Life
Uploaded by
Misty VitoThis document discusses half-life, which is the time it takes for half of the atoms in a radioactive sample to decay. It provides examples of half-lives for various radioisotopes ranging from 5 years to 4.5 billion years. Uranium-238 has a very long half-life of 4.5 billion years and is used for geological dating. The oldest rocks on Earth are around 3.7 billion years old based on uranium-238 dating. Several examples are given to illustrate how to calculate the amount of radioactive material remaining after a given number of half-lives. The rate of radioactive decay, characterized by half-life, remains constant regardless of external conditions like pressure and temperature.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 8
HALF-LIFE
Length
of time during which
half of a given number of
atoms of a radioactive
nuclide decays.
An isotope with long half-life
is stable. An isotope with
very short half-life is very
radioactive.
Half-lives of Several Radioisotopes
Isotope:
Half-life:
Use:
5 years
14 days
Chemothe
detect
tumors
rapy
5730
years
4.5 billion
8 days
years
Archeolo
Hyperthyroi
gical dating
dism
geological
dating
NOTE: has a half-life of 4.5 billion
years. Very old rock samples can be
dated based on their
content.
The
oldest rock found on earth (in
Greenland) was dated 3.7 billion
years. The solar system has an
estimated age of 4.6 billion years
based on dating meteorites
Ex. The half-life of radiogold is approximately
three days. Radiogold is given to a patient
undergoing cancer therapy. Suppose a patient
is given an initial shot of 1.5 g of radiogold,
how much of it remains after nine days?
Time (days)
0
3
6
9
Amount (grams)
1.5
0.75
0.375
0.1875
Thus, only 0.1875 g remains after 9 days.
Barium-122 has a half-life of 2 minutes. A fresh
sample weighing 80 g was obtained. If it takes 10
minutes to set up an experiment using barium122, how much barium-122 will be left when the
experiment begins?
Every half-life, 2 minutes, half of the original
amount will undergo nuclear decay:
10
Time: start 2 min 4 min 6 min 8 min
min
Mass:
80 g
40 g
20 g
10 g
5g
2.5 g
At the end of 10 minutes (5 half-lives) only2.5 gare left,
the rest has decayed.
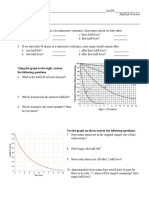
Exercise
If 10 mg of iodine 131 is given to a patient,
how much is left after 24 days? The half-life
of iodine-131 is 8 days.
Iodine-131 is used to destroy thyroid tissue
in the treatment of an overactive thyroid.
The half-life of iodine-131 is 8 days. If a
hospital receives a shipment of 200 g of
iodine-131, how much I-131 would remain
after 32 days?
1.
The half life of a certain isotope is
1 day. At the end of two days, how
much of the isotope remains?
2. A sample of certain radioactive
material has a half-life of 1 year. How
much of this radioactive material will
be left at the end of three years?
Rates of radioactivity are remarkably
constant and are not affected by any
external conditions such as changes in
pressure and temperature.
You might also like
- Half Life Worksheet - Problems and GraphingDocument2 pagesHalf Life Worksheet - Problems and GraphingAamir HamzahNo ratings yet
- 29.half Life ConceptDocument30 pages29.half Life ConceptAnonymous UypCttWNo ratings yet
- Class # - : Radiometric Dating Practice Name: - Core 1 2 3Document3 pagesClass # - : Radiometric Dating Practice Name: - Core 1 2 3Alvin PaboresNo ratings yet
- Bio102 Assignment 6Document3 pagesBio102 Assignment 6Daniel50% (2)
- Radioactive Decay WorksheetDocument4 pagesRadioactive Decay WorksheetSuta PinatihNo ratings yet
- Nuclear MedicineDocument7 pagesNuclear MedicineMarco Mendoza67% (3)
- Answerkey 3Document52 pagesAnswerkey 3Gleydi Zetino-Moreno50% (2)
- Half Life ProblemsDocument2 pagesHalf Life ProblemsPinak BawankarNo ratings yet
- Radioactivity and Half-LifeDocument16 pagesRadioactivity and Half-Life애라 김 Ae RaNo ratings yet
- 060 Half Life WorksheetDocument3 pages060 Half Life WorksheetLin Xian XingNo ratings yet
- W Radioactivityandhalflives answERKEYDocument2 pagesW Radioactivityandhalflives answERKEYreed99951No ratings yet
- Earth Science Week 4 - Q2Document4 pagesEarth Science Week 4 - Q2Giane MadrigalNo ratings yet
- CH 10 Nuclear ChemistryDocument40 pagesCH 10 Nuclear ChemistryBùi Thảo LyNo ratings yet
- Radioactivity and Half-LifeDocument19 pagesRadioactivity and Half-LifeJustin Dellomas100% (1)
- HALF-life QuestionsDocument1 pageHALF-life QuestionsNii LaryeaNo ratings yet
- Absolute DatingDocument20 pagesAbsolute DatingJorilyn LlandaNo ratings yet
- Fossil Power Point PresentationDocument25 pagesFossil Power Point PresentationSophia OrtizNo ratings yet
- 7 3 NotesDocument6 pages7 3 Notesapi-182809945No ratings yet
- Radioactivity 1Document25 pagesRadioactivity 1Kate GanalonNo ratings yet
- Nama: Muhamad Khairi Asyraf Bin Basri KELAS: 503Document21 pagesNama: Muhamad Khairi Asyraf Bin Basri KELAS: 503Aiman KpNo ratings yet
- Gambaran Makroskopik Dan Mikroskopik Organ Tiroid Pada Hewan Coba PostmortemDocument7 pagesGambaran Makroskopik Dan Mikroskopik Organ Tiroid Pada Hewan Coba PostmortemArda NaufalNo ratings yet
- Radio Active Decay and Half Life PracticeDocument4 pagesRadio Active Decay and Half Life PracticeEmmalee ThomsenNo ratings yet
- Half Life Reactions Discussions and SolutionsDocument20 pagesHalf Life Reactions Discussions and SolutionsDeiparineIrisNo ratings yet
- Half Life Worksheet With AnswersDocument6 pagesHalf Life Worksheet With AnswersAnbiya FathimaNo ratings yet
- RadioactivityDocument31 pagesRadioactivityRamliRemNo ratings yet
- DS-4, English MediumDocument56 pagesDS-4, English MediumRashini AnneNo ratings yet
- Nuclear ChemistryDocument47 pagesNuclear ChemistryEmman Revilla100% (3)
- Science 10 Q2 W3 D2Document15 pagesScience 10 Q2 W3 D2AntonNo ratings yet
- HalflivesDocument18 pagesHalflivesapi-252814424No ratings yet
- There Are Three Types of RadiationDocument6 pagesThere Are Three Types of RadiationJoseph ZhangNo ratings yet
- Isotopes, The Siblings of AtomsDocument3 pagesIsotopes, The Siblings of AtomsFatafut ShoppingNo ratings yet
- Radioactivity and Half-Life From GigaPhysicsDocument2 pagesRadioactivity and Half-Life From GigaPhysicsAlistair DaleyNo ratings yet
- Half Life Worksheet - Problems and GraphingDocument2 pagesHalf Life Worksheet - Problems and GraphingAamir HamzahNo ratings yet
- NW6Document10 pagesNW6Mamidala HarithaNo ratings yet
- Absolute DatingDocument24 pagesAbsolute DatingMelanie CalladaNo ratings yet
- Half Life Practice SDocument3 pagesHalf Life Practice SLÂM VŨ THÙYNo ratings yet
- UntitledDocument36 pagesUntitledahsan shahNo ratings yet
- Notes Chemistry 1 201 300Document100 pagesNotes Chemistry 1 201 300c0ldh337No ratings yet
- Half-Life Student Lab Sheet-REVDocument6 pagesHalf-Life Student Lab Sheet-REVElPerritoSapeeNo ratings yet
- 4.3, 4.4, 4.5 Radiation Measurement HL MAppDocument15 pages4.3, 4.4, 4.5 Radiation Measurement HL MAppIvy Joyce BuanNo ratings yet
- 5 RadioactivityDocument22 pages5 RadioactivityreubenNo ratings yet
- Avhusifhxm &RNFH Tcef (13) Ar Cgef A (Mif Twgufykpäm/: DR Vince Grade 10 Physics: Questions and ProblemsDocument2 pagesAvhusifhxm &RNFH Tcef (13) Ar Cgef A (Mif Twgufykpäm/: DR Vince Grade 10 Physics: Questions and ProblemsPyae Sone KyawNo ratings yet
- Absolute DatingDocument16 pagesAbsolute DatingDumagpi John Mark A.No ratings yet
- Time of Death: Method For DeterminingDocument32 pagesTime of Death: Method For DetermininglilaNo ratings yet
- RadioactivityHalflife 05.09.2021Document25 pagesRadioactivityHalflife 05.09.2021Erdem AltunNo ratings yet
- BiochemistryDocument27 pagesBiochemistryKyle BroflovskiNo ratings yet
- Half Life HWDocument2 pagesHalf Life HWLiza Cabalquinto LorejoNo ratings yet
- Absolute Dating Problem SetDocument9 pagesAbsolute Dating Problem SetjeannythethirdNo ratings yet
- 100% Efforts With Consistency Date: 24th Apiril 2020 30 Days Plan Last Date: 23rd May 2020Document12 pages100% Efforts With Consistency Date: 24th Apiril 2020 30 Days Plan Last Date: 23rd May 2020guru charanNo ratings yet
- Biology SPM Quick RevisionDocument37 pagesBiology SPM Quick RevisionAsyraf GhaniNo ratings yet
- IGCSE Revision BookDocument67 pagesIGCSE Revision BookTim FiltnessNo ratings yet
- Worksheet Half LifeDocument3 pagesWorksheet Half LifekiranNo ratings yet
- RadioactivityDocument31 pagesRadioactivitysanaNo ratings yet
- Radiopharmaceuticals : PROF - SANDIP MAVCHI..7506929220Document47 pagesRadiopharmaceuticals : PROF - SANDIP MAVCHI..7506929220Arvind pharmacy groupNo ratings yet
- Everything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksFrom EverandEverything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksNo ratings yet
- Science Form 1 NotesDocument37 pagesScience Form 1 Notespuviniyaraj100% (1)
- 05 - Half-Life & Radioisotope DatingDocument19 pages05 - Half-Life & Radioisotope DatingNawraa AldaifNo ratings yet
- Forensics U4 Bio AlevelDocument6 pagesForensics U4 Bio AlevelNMVRNo ratings yet
- PlagiarismDocument2 pagesPlagiarismMisty VitoNo ratings yet
- Cat MusclesDocument5 pagesCat MusclesMisty VitoNo ratings yet
- Electromagnetic SpectrumDocument50 pagesElectromagnetic SpectrumMisty Vito100% (1)
- Logic Gates: Logic 1. Logic 0. Logic Truth TableDocument16 pagesLogic Gates: Logic 1. Logic 0. Logic Truth TableMisty Vito100% (1)