Professional Documents
Culture Documents
Kinetic Chemistry - Equation
Kinetic Chemistry - Equation
Uploaded by
Irsyad Kamil0 ratings0% found this document useful (0 votes)
6 views2 pagesThis document summarizes reaction order, rate laws, integrated rate laws, half lives, units of rate constants, and the Arrhenius equation. It shows the equations for zero-order, first-order, and second-order reactions, including plots of concentration versus time. The half life is defined as the time required for the concentration to reduce to half the initial value. The Arrhenius equation relates the rate constant to temperature.
Original Description:
Chemistry
Original Title
Kinetic Chemistry- Equation
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document summarizes reaction order, rate laws, integrated rate laws, half lives, units of rate constants, and the Arrhenius equation. It shows the equations for zero-order, first-order, and second-order reactions, including plots of concentration versus time. The half life is defined as the time required for the concentration to reduce to half the initial value. The Arrhenius equation relates the rate constant to temperature.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
6 views2 pagesKinetic Chemistry - Equation
Kinetic Chemistry - Equation
Uploaded by
Irsyad KamilThis document summarizes reaction order, rate laws, integrated rate laws, half lives, units of rate constants, and the Arrhenius equation. It shows the equations for zero-order, first-order, and second-order reactions, including plots of concentration versus time. The half life is defined as the time required for the concentration to reduce to half the initial value. The Arrhenius equation relates the rate constant to temperature.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 2
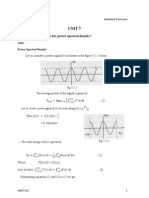
Reaction
Order
Rate Law
Integrated Rate Law
Half life
Units of
Rate Constant
Linear Plot
y = mx + C
[A]t = - kt + [A]0
[]
=k
[A]0
[A] = [A]0 - kt
t =
[]0
2
M s -1
[A]t
y = mx + C
In [A]t = - kt + In [A]0
[]
= k [A]
In [A] = -kt + In[A]0
t =
In [A]0
s -1
In
[A]t
y = mx + C
1
[]
= k [A]2
1
[]
= kt +
[]
= kt +
1
[]0
[]0
1
t = []
M -1 s -1
1
[]
1
[]0
t
y = mx + C
1
In k =
+ In
Arrhenius
equation
K=
In k =
In
2
1
+ In A
1
2
In A
In k
0 order
0 order
[A]
Rate
(M/s)
nd
1st order
2 order
t
1st order
2ndorder
You might also like
- ECEN 619: Internet Protocols & Modeling: Sridhar Mareguddi April 16, 2015 UIN: 823000772Document7 pagesECEN 619: Internet Protocols & Modeling: Sridhar Mareguddi April 16, 2015 UIN: 823000772Sanjiti BhargavaNo ratings yet
- Modern Control Project FinalDocument45 pagesModern Control Project Finalyohannes100% (1)
- CSM Chapters12 PDFDocument29 pagesCSM Chapters12 PDFClau AmaiiaNo ratings yet
- Robin AssignmentDocument29 pagesRobin AssignmentMoon Yu Jie100% (1)
- DocumentDocument11 pagesDocumentAbane Jude yenNo ratings yet
- Differential and Integrated Rate Laws: K DT D (A) DT K D (A) DT K D (A) T K (A) - (A) (T) T K (A) (A) (T)Document6 pagesDifferential and Integrated Rate Laws: K DT D (A) DT K D (A) DT K D (A) T K (A) - (A) (T) T K (A) (A) (T)Mahmoud AyadNo ratings yet
- Chapter 2-Mass Reactor Model (102 P)Document102 pagesChapter 2-Mass Reactor Model (102 P)shardulkaviNo ratings yet
- Kalman SmoothingDocument15 pagesKalman SmoothingMarisela BarnesNo ratings yet
- Chemical Kinetics: Peter Atkins, Physical Chemistry, 7 EditionDocument38 pagesChemical Kinetics: Peter Atkins, Physical Chemistry, 7 EditionalchemiyNo ratings yet
- Kalman Filter and Parameter Identification: Florian Herzog 2013Document35 pagesKalman Filter and Parameter Identification: Florian Herzog 2013Dragan LazicNo ratings yet
- 55-2-2 PhysicsDocument16 pages55-2-2 PhysicsK_S_Krishna0001No ratings yet
- Derivation and Log-Linearization of Otsu (2007) 'S Small Open Economy ModelDocument46 pagesDerivation and Log-Linearization of Otsu (2007) 'S Small Open Economy ModelkeyyongparkNo ratings yet
- Chapter 2 Mathematics - Class 12 - Formula - SheetDocument3 pagesChapter 2 Mathematics - Class 12 - Formula - SheetAditya SharmaNo ratings yet
- Three Period OLGDocument12 pagesThree Period OLGDavidChum Lai100% (1)
- Process Dynamics and Control, Ch. 8 Solution ManualDocument12 pagesProcess Dynamics and Control, Ch. 8 Solution ManualBen SpearmanNo ratings yet
- 55-2-3 PhysicsDocument16 pages55-2-3 PhysicsK_S_Krishna0001No ratings yet
- Math Formula ListDocument3 pagesMath Formula ListKumaravel PadmaroopaNo ratings yet
- Answer Key: Paper-2Document14 pagesAnswer Key: Paper-2vishal110085No ratings yet
- Z TransformDocument22 pagesZ Transformvignanaraj100% (1)
- CH 1 PDFDocument43 pagesCH 1 PDFVimala ElumalaiNo ratings yet
- CDS IiDocument48 pagesCDS IiTalha YasinNo ratings yet
- Instituto Tecnologico Superios de Martinez de La TorreDocument7 pagesInstituto Tecnologico Superios de Martinez de La TorreBraulio CuevasNo ratings yet
- Te Techn Ical R Repo RT: Uti Lizatio On of Fract Gron Tional Wall-B Orde Bellm Er Sys Man Le Stems Emma S AinDocument5 pagesTe Techn Ical R Repo RT: Uti Lizatio On of Fract Gron Tional Wall-B Orde Bellm Er Sys Man Le Stems Emma S AinkarpagasenthilpandyNo ratings yet
- Instantaneous Frac. Yield Overall Fractional Yield: Max. Mix. ModelDocument2 pagesInstantaneous Frac. Yield Overall Fractional Yield: Max. Mix. ModelElena TodorovskaNo ratings yet
- Vibration and Control: Associate Professor Department of Mechanical Engineering Y.T.UDocument82 pagesVibration and Control: Associate Professor Department of Mechanical Engineering Y.T.Udora901No ratings yet
- Chemical Kinetics1Document59 pagesChemical Kinetics1farooq_bagbanNo ratings yet
- 6 Hansen ModelDocument26 pages6 Hansen ModelSemíramis LimaNo ratings yet
- Adaptive Control Theory: Pole-Placement and Indirect STRDocument48 pagesAdaptive Control Theory: Pole-Placement and Indirect STRThanh NguyenNo ratings yet
- Códigos Proyecto - Termodinámica Del EquilibrioDocument32 pagesCódigos Proyecto - Termodinámica Del EquilibriojuangarciaNo ratings yet
- Time Domain Analysis of 1st Order SystemsDocument19 pagesTime Domain Analysis of 1st Order SystemsIslam SaqrNo ratings yet
- 1 Math Review: 2.1 Basic DefinitionsDocument6 pages1 Math Review: 2.1 Basic DefinitionsMia GheeNo ratings yet
- Peretmuan 12 Laplace in CircuitsDocument56 pagesPeretmuan 12 Laplace in CircuitsSando CrisiasaNo ratings yet
- 210 Nice Symmetric InequalitiesDocument7 pages210 Nice Symmetric InequalitiesswapnilNo ratings yet
- Problem Set 2 Sample AnswersDocument10 pagesProblem Set 2 Sample Answersjc224No ratings yet
- MIT6 003S10 Lec04 PDFDocument62 pagesMIT6 003S10 Lec04 PDFThắng PyNo ratings yet
- Systems of Surveillance of Dynamic ProcessesDocument13 pagesSystems of Surveillance of Dynamic ProcessesnickNo ratings yet
- Michal Iz Ak, Daniel G Orges and Steven Liu (Izak - Goerges - Sliu@eit - Uni-Kl - De) Institute of Control Systems, University of Kaiserslautern, GermanyDocument1 pageMichal Iz Ak, Daniel G Orges and Steven Liu (Izak - Goerges - Sliu@eit - Uni-Kl - De) Institute of Control Systems, University of Kaiserslautern, GermanyAbbas AbbasiNo ratings yet
- Derive The Integrated Rate Equation Half-LifeDocument7 pagesDerive The Integrated Rate Equation Half-Lifeumut2000No ratings yet
- Discrete-Time Signals and SystemsDocument111 pagesDiscrete-Time Signals and Systemsduraivel_anNo ratings yet
- Models of Industrial Control Devices and Systems: R G G Y G+G + + +Document12 pagesModels of Industrial Control Devices and Systems: R G G Y G+G + + +陳瑞祥No ratings yet
- Unit 7: 1. Derive An Expression For Power Spectral Density? AnsDocument11 pagesUnit 7: 1. Derive An Expression For Power Spectral Density? AnssrinivasNo ratings yet
- Line IntersectionDocument1 pageLine IntersectionCat AterNo ratings yet
- Kalman Filtering and LQG Control - Acs10-12 PDFDocument37 pagesKalman Filtering and LQG Control - Acs10-12 PDFTranNhanNo ratings yet
- Ee263 Ps1 SolDocument11 pagesEe263 Ps1 SolMorokot AngelaNo ratings yet
- Exam MLC FormulasDocument6 pagesExam MLC Formulasnight_98036No ratings yet
- Z TransformDocument22 pagesZ TransformcivaasNo ratings yet
- Lecture 4 First Order Systems: AME 455 Control Systems DesignDocument22 pagesLecture 4 First Order Systems: AME 455 Control Systems DesignJason ChiangNo ratings yet
- Discrete-Time Signals and SystemsDocument111 pagesDiscrete-Time Signals and SystemsharivarahiNo ratings yet
- V T) =3 Cosωt=3 ∠0 °=3+0 J V T) =2 Cosωt=2 ∠0 °=2+0 J X J Ωt J X Ωlj= (2 Π) (100 Hz) (5 Mh) =3.14159 JωDocument2 pagesV T) =3 Cosωt=3 ∠0 °=3+0 J V T) =2 Cosωt=2 ∠0 °=2+0 J X J Ωt J X Ωlj= (2 Π) (100 Hz) (5 Mh) =3.14159 JωAlicia MagañaNo ratings yet
- Appendix A.: Res ResDocument5 pagesAppendix A.: Res ResYanira Clavijo HernandezNo ratings yet
- Formula Rio de As HorarioDocument1 pageFormula Rio de As HorarioRamón Moreno MirandaNo ratings yet
- Modern Control Lec2Document19 pagesModern Control Lec2Saad FaruquiNo ratings yet
- Calculations Estimation Parameter: Block Diagram of A Self Tuning RegulatorDocument10 pagesCalculations Estimation Parameter: Block Diagram of A Self Tuning Regulatorvk2you009No ratings yet
- State Space ModelsDocument8 pagesState Space ModelsRamanan MuthuramanNo ratings yet
- Analytic Geometry: Graphic Solutions Using Matlab LanguageFrom EverandAnalytic Geometry: Graphic Solutions Using Matlab LanguageNo ratings yet
- Inverse Trigonometric Functions (Trigonometry) Mathematics Question BankFrom EverandInverse Trigonometric Functions (Trigonometry) Mathematics Question BankNo ratings yet
- Tutorial 2 StudentDocument6 pagesTutorial 2 StudentIrsyad KamilNo ratings yet
- Chm096 Chapter 2 Chemical Kinetics Nov 2013 - Mac 2014Document167 pagesChm096 Chapter 2 Chemical Kinetics Nov 2013 - Mac 2014Irsyad KamilNo ratings yet
- Carboxylic Acids and Esters and Amines New Edition Chm096Document26 pagesCarboxylic Acids and Esters and Amines New Edition Chm096Irsyad KamilNo ratings yet
- Reaction PH Between Acid & Bases Acid Base PH Formula Strong Acid (SA) Strong Base (SB)Document4 pagesReaction PH Between Acid & Bases Acid Base PH Formula Strong Acid (SA) Strong Base (SB)Irsyad KamilNo ratings yet