Professional Documents
Culture Documents
Ccb3043 Kinetics and Reactor Design (January 2015) : Tutorial 1
Ccb3043 Kinetics and Reactor Design (January 2015) : Tutorial 1
Uploaded by
minumcincauOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ccb3043 Kinetics and Reactor Design (January 2015) : Tutorial 1
Ccb3043 Kinetics and Reactor Design (January 2015) : Tutorial 1
Uploaded by
minumcincauCopyright:
Available Formats
CCB3043 KINETICS AND REACTOR DESIGN (January 2015)
Tutorial 1
1. The irreversible gas phase non-elementary reaction, A + 2B C, is to be
carried out isothermally in a constant-pressure batch reactor. The feed is at a
temperature of 227oC, and pressure of 1013 kPa. The composition of the feed
is 33.3% A and 66.7% B. The lab data taken under identical condition is as
follows:
-rA (mol/dm3.min) x
103
X
0.01
0.005
0.002
0.001
0.0
0.2
0.4
0.6
a. Estimate the volume of plug flow reactor required for 30% conversion of A
for entering volumetric flow rate of 2 m3/min
b. Estimate the volume of the CSTR required to take the effluent from the
plug flow reactor in part (a) to achieve the final total conversion of 50%.
c. Plot the graph of reaction rate and conversion as a function of PFR volume.
Comment on the graph profile that you have obtained.
[P2-8, C4, C5]
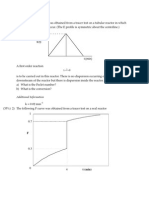
2. The figure below represents a typical gas-solid catalytic exothermic reaction
carried out adiabatically. Assuming that you have a fluidized CSTR and a PBR
containing catalyst, suggest a suitable reactor arrangement to achieve 80%
conversion using the smallest amount of catalyst. Determine the weight of
catalyst required in each reactor.
[P2-12, C5]
CCB3043 KINETICS AND REACTOR DESIGN (January 2015)
You might also like
- Assignment Aspen PlusDocument8 pagesAssignment Aspen PlusVinayak PathakNo ratings yet
- Chemical Reaction Engineering Exercise One 2022Document4 pagesChemical Reaction Engineering Exercise One 2022Matone MafologelaNo ratings yet
- Revision QuestionsDocument12 pagesRevision QuestionsLiew Wen Xuan0% (2)
- Chapter 2Document16 pagesChapter 2Awat MuhammadNo ratings yet
- MD2 SolutionDocument6 pagesMD2 SolutionA.Kh.SNo ratings yet
- CDP2 DDocument1 pageCDP2 Dcast93No ratings yet
- CHE 502 Tutorial 5Document3 pagesCHE 502 Tutorial 5Ibnu HamidNo ratings yet
- CDB2043 - Reaction EngineeringDocument6 pagesCDB2043 - Reaction EngineeringXin-YiWoonNo ratings yet
- 5895223Document14 pages5895223DeneshVijayNo ratings yet
- Tutorial QuestionsDocument8 pagesTutorial QuestionsMaame Efua Neizer100% (1)
- Tutorial 5drtuhDocument2 pagesTutorial 5drtuhFikrie MuhdNo ratings yet
- Quizzes 07solDocument15 pagesQuizzes 07solBeto MelgarejoNo ratings yet
- (P01, C01, C02, C2, C3) : Confidential EH/JUN 2014/CHE584/594Document11 pages(P01, C01, C02, C2, C3) : Confidential EH/JUN 2014/CHE584/594Addison JuttieNo ratings yet
- Chapter 3 - ExerciseDocument24 pagesChapter 3 - ExerciseNguyễn Văn HòaNo ratings yet
- DQE January 2001: Additional InformationDocument12 pagesDQE January 2001: Additional InformationryezhuNo ratings yet
- Tutorial 3 QuestionDocument3 pagesTutorial 3 Questionnur hidayatiNo ratings yet
- Department of Chemical Engineering, Iit Delhi Reactor Sizing Problems Assignment-2Document2 pagesDepartment of Chemical Engineering, Iit Delhi Reactor Sizing Problems Assignment-2ShubhamGuptaNo ratings yet
- Tutorial For Chapter 23Document9 pagesTutorial For Chapter 23Thurgah VshinyNo ratings yet
- University of Mauritius University of Mauritius University of Mauritius University of MauritiusDocument4 pagesUniversity of Mauritius University of Mauritius University of Mauritius University of MauritiusToMemNo ratings yet
- Essy Questions On Non-Ideal ReactorsDocument9 pagesEssy Questions On Non-Ideal ReactorsRobinson ANo ratings yet
- Tutorial3 - C3 - CHE 244Document3 pagesTutorial3 - C3 - CHE 244Sheikh AdibNo ratings yet
- CKB 20104 - Reaction EngineeringDocument9 pagesCKB 20104 - Reaction EngineeringNoor FatihahNo ratings yet
- Revision QuestionDocument2 pagesRevision QuestionBilal AhmadNo ratings yet
- Chương 1 - Bài TậpDocument25 pagesChương 1 - Bài TậpTÍN Phạm Nguyễn TrọngNo ratings yet
- NH H N: T (Min) CDocument1 pageNH H N: T (Min) CPIratȅ IlıNo ratings yet
- Tut1 2016 QDocument5 pagesTut1 2016 QAbhishek SardaNo ratings yet
- Thermo OldStylePastPaper 2007-19Document54 pagesThermo OldStylePastPaper 2007-19manjeet gajbhiyeNo ratings yet
- Problem Set 10 - GeneralDocument7 pagesProblem Set 10 - GeneralAdekoya IfeoluwaNo ratings yet
- Microsoft Word - 6 - Prob RTD-Non Id React 11-12 61-78 - EnglishDocument9 pagesMicrosoft Word - 6 - Prob RTD-Non Id React 11-12 61-78 - EnglishPavithra Sivaraja100% (1)
- Chemical Reactors - Problems of Reactor Association 47-60: (Exam Jan'09)Document6 pagesChemical Reactors - Problems of Reactor Association 47-60: (Exam Jan'09)Alfredo ZuñigaNo ratings yet
- CHE3044F, 2013: Reactor Design 1: TUTORIAL 6Document4 pagesCHE3044F, 2013: Reactor Design 1: TUTORIAL 6nmhatityeNo ratings yet
- Exam I: Chemical Reactor Design (CHEG 322)Document6 pagesExam I: Chemical Reactor Design (CHEG 322)Faisal MumtazNo ratings yet
- Example 7.1Document9 pagesExample 7.1YULISSA MARCELA RODRIGUEZ CARDENASNo ratings yet
- 9A23502 Biochemical Reaction Engineering IDocument8 pages9A23502 Biochemical Reaction Engineering IsivabharathamurthyNo ratings yet
- Compulsory Question: Question (1) : 10 Marks: A B C DDocument4 pagesCompulsory Question: Question (1) : 10 Marks: A B C Dsushant mouleNo ratings yet
- Introduction To Chemical Reactor Engineering - Problems PDFDocument75 pagesIntroduction To Chemical Reactor Engineering - Problems PDFJojie-Ann Alabarca100% (1)
- Ki KBR H C Ki BR H C: Oducts B ADocument2 pagesKi KBR H C Ki BR H C: Oducts B AnaverfallNo ratings yet
- Cre 1 Solution PDFDocument21 pagesCre 1 Solution PDFSaints Burner Christopher100% (1)
- Press ReleaseDocument3 pagesPress ReleaseJuanita López SánchezNo ratings yet
- Unsw Sydney School of Chemical Engineering Sample Paper Ceic 2005 Chemical Reaction EngineeringDocument6 pagesUnsw Sydney School of Chemical Engineering Sample Paper Ceic 2005 Chemical Reaction EngineeringJoshua JohnNo ratings yet
- HW 2 K 1606Document7 pagesHW 2 K 1606Primus OngNo ratings yet
- Ideal Reactors Part 2 Solved ProblemsDocument15 pagesIdeal Reactors Part 2 Solved Problemschandankumar356500000012No ratings yet
- Ideal Reactors Part 2 Solved ProblemsDocument15 pagesIdeal Reactors Part 2 Solved ProblemsWaldi SagalaNo ratings yet
- 3 - Prob PFR 11-12 23-35 English-1Document4 pages3 - Prob PFR 11-12 23-35 English-1Biniyam haileNo ratings yet
- Tutorial 2 QuestionDocument3 pagesTutorial 2 Questionnur hidayatiNo ratings yet
- Or Nek FinalDocument3 pagesOr Nek Finalcinarsercan_69627706No ratings yet
- Final QuestionsDocument6 pagesFinal QuestionsrushdiNo ratings yet
- Question No. 2 (30 Marks) (4+4+9+5+8) : E) For The Following Statements, Explain Briefly Why?Document2 pagesQuestion No. 2 (30 Marks) (4+4+9+5+8) : E) For The Following Statements, Explain Briefly Why?أحمد إبراهيم شواربNo ratings yet
- E 199 SolDocument10 pagesE 199 SoltacoNo ratings yet
- CHE3044F, 2013: Reactor Design 1: TUTORIAL 5Document2 pagesCHE3044F, 2013: Reactor Design 1: TUTORIAL 5nmhatityeNo ratings yet
- r05310805 Chemical Reaction Engineering IDocument8 pagesr05310805 Chemical Reaction Engineering ISrinivasa Rao GNo ratings yet
- Bkf3472 - Chem. Reaction Engineering II 21616-2Document4 pagesBkf3472 - Chem. Reaction Engineering II 21616-2Siti HajarNo ratings yet
- CL324 - 2023 - Tutorial 02Document2 pagesCL324 - 2023 - Tutorial 02Prince KumarNo ratings yet
- Exercise TRK 1Document14 pagesExercise TRK 1Ananda CahyaNo ratings yet
- Partial ExamDocument1 pagePartial ExamFranco CamachoNo ratings yet
- Tutorial For Chapter 1Document3 pagesTutorial For Chapter 1Thurgah VshinyNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Patent Office: United StatesDocument2 pagesPatent Office: United StatesminumcincauNo ratings yet
- Cellulose Nanocrystals: Synthesis, Functional Properties, and ApplicationsDocument10 pagesCellulose Nanocrystals: Synthesis, Functional Properties, and ApplicationsminumcincauNo ratings yet
- Nano Cellulose SDocument3 pagesNano Cellulose SminumcincauNo ratings yet
- Drinking Water: Professional Communication SkillsDocument9 pagesDrinking Water: Professional Communication SkillsminumcincauNo ratings yet
- How To Prepare Robot For Robotic CompetitionDocument19 pagesHow To Prepare Robot For Robotic CompetitionminumcincauNo ratings yet
- Lee - Lead Acid, LiPoDocument12 pagesLee - Lead Acid, LiPominumcincauNo ratings yet
- Chocolate PresentationDocument9 pagesChocolate PresentationminumcincauNo ratings yet
- Student Name ID: Aysha Housani 200503484 Maha Al Shehhi 200509462 Hessa Al Shehhi 200509582 Mona Thabet 200521150Document78 pagesStudent Name ID: Aysha Housani 200503484 Maha Al Shehhi 200509462 Hessa Al Shehhi 200509582 Mona Thabet 200521150minumcincauNo ratings yet