Professional Documents
Culture Documents
Daily Practice Problem Sheet-1: (Alkanes)
Daily Practice Problem Sheet-1: (Alkanes)
Uploaded by
abhijeet_sangwanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Daily Practice Problem Sheet-1: (Alkanes)
Daily Practice Problem Sheet-1: (Alkanes)
Uploaded by
abhijeet_sangwanCopyright:
Available Formats
Daily Practice Problem Sheet-1
(Alkanes)
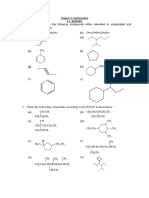
1. What is the IUPAC name for CH3CH2C(CH3)2CH2CH(CH3)2?
(a) 3,3,5-trimethylhexane
(b) 2,2,5-trimethylhexane
(c) 2,4,4-trimethylhexane
(d) 1,1,3,3-tetramethylpentane
2. The radical halogenation of 2-methylpropane gives two products: (CH 3)2CHCH2X (minor) and (CH3)3CX
(major). Chlorination gives a larger amount of the minor product than does bromination, Why?
(a) Bromine is more reactive than chlorine and is able to attack the less reactive 3 C-H.
(b) Bromine atoms are less reactive (more selective) than chlorine, and preferentially attack the weaker 3
C-H bond.
(c) The methyl groups are more hindered to attack by the larger bromine atom.
(d) Bromination is reversible and the more stable 3-alkyl bromide is formed exclusively.
3. How many dichlorinated isomers can be formed by the halogenation of CH 3CH2CH2CH3 with Cl2 in the
presence of light?
(a) 2
(b) 3
(c) 5
(d) 6
4. A sec-butyl group is?
(a) (CH3)4C
(b) (CH3)3C(c) (CH3)2CHCH2(d) CH3CH2CH(CH3)5. A chiral C6H12 hydrocarbon undergoes catalytic hydrogenation to yield an achiral C6H14 product. What
is the starting compound?
(a) cis-2-hexene
(b) 3-methyl-2-pentene
(c) 4-methyl-2-pentene
(d) 3-methyl-1-pentene
6. The highest boiling point is expected for
(a) Isooctane
(b) n-octane
(c) 2,2,3,3-tetramethylbutane (d) n-butane
7. In which of the following reactions a new C C bond is formed
(a) Sabatier-Sandrens reaction
(b) Wurtz reaction
(c) Clemmensen reduction
(d) Wolf-kishner reduction
8. Propose synthesis of n-hexane from each of the following
(a) CH3CH2CH2CH2CH2CH2Br
(b) CH3CH=CHCH2CH2CH3
(c) CH3CH2CH2Br
(d) CH3CH2CH2CH2CH2CHO
9. Propose a synthesis of CH3D from CH4
10. Arrange the following in order of decreasing melting point
(a) Octane, Hexane and decane,
(b) n-octane, 3,3-dimethylhexane and 2,2,3,3 tetramethylbutane
11. Write equations for the preparation of n-butane from
(a) n-butyl bromide
(b) sec-butyl bromide (c) ethyl chloride
(d) 1-butene
(e) 2-butene
12. draw structures of all products expected from monochlorination at room temp of
(a) n-hexane
(b) iso-hexane
(c) 2,2,4-trimethylpentane
(d) 2,2-dimethylbutane
You might also like
- Organic Chemistry 8th Edition Wade Test Bank DownloadDocument35 pagesOrganic Chemistry 8th Edition Wade Test Bank DownloadChelsea Mathur100% (18)
- Org Chem 3Document37 pagesOrg Chem 3tyron9520100% (1)
- Organic Chemistry 1 Multiple Choice: Cis TransDocument4 pagesOrganic Chemistry 1 Multiple Choice: Cis Transacb4039No ratings yet
- Daily Practice Problem Sheet-1: (Alkanes)Document1 pageDaily Practice Problem Sheet-1: (Alkanes)Rajeev GangwarNo ratings yet
- 01 HydrocarbonsDocument6 pages01 HydrocarbonslingarajugowdaNo ratings yet
- Alkanes Alkenes AlkynesDocument10 pagesAlkanes Alkenes AlkynesPanda Boy100% (2)
- A (2e, 4e) B (2Z, 4Z) C (2Z, 4e) D (2e, 4Z)Document1 pageA (2e, 4e) B (2Z, 4Z) C (2Z, 4e) D (2e, 4Z)Agatha chilesheNo ratings yet
- Alka NetDocument13 pagesAlka Netjonida88No ratings yet
- Tutor 1 Kimia PDFDocument3 pagesTutor 1 Kimia PDFFaris 2806No ratings yet
- Alkanes, Alkenes, Alkynes and Their Alicyclic Couterparts: 1. What Is The IUPAC Name For CHDocument17 pagesAlkanes, Alkenes, Alkynes and Their Alicyclic Couterparts: 1. What Is The IUPAC Name For CHEllaŠtrbac100% (1)
- VPTS-3B 18-03-2021Document7 pagesVPTS-3B 18-03-2021Aayush NagpalNo ratings yet
- Alkyl Halides INTRO WS - SV - Spring 2023Document2 pagesAlkyl Halides INTRO WS - SV - Spring 2023Shonte ScottNo ratings yet
- Wade Ch.4 QuestionsDocument8 pagesWade Ch.4 QuestionsAlexGeorgeNo ratings yet
- Test of Chemistry: Class: 2 Year Total Marks: 50 Time Limit: 100min Q.1Document1 pageTest of Chemistry: Class: 2 Year Total Marks: 50 Time Limit: 100min Q.1Shah JahanNo ratings yet
- Haloalkanes & Haloarenes - 15-11-2020Document45 pagesHaloalkanes & Haloarenes - 15-11-2020Class 11aNo ratings yet
- NCERT Solutions For Class 12 Chemistry Chapter 10 Haloalkanes and HaloarenesDocument39 pagesNCERT Solutions For Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenesabhik525No ratings yet
- Orgsynth KeysDocument27 pagesOrgsynth KeysDebalina DassNo ratings yet
- Chapter 03Document40 pagesChapter 03AC BañaresNo ratings yet
- Organic Chemistry QuestionsDocument4 pagesOrganic Chemistry QuestionsqawiyamenimbabyNo ratings yet
- OCHEM Practice FinalsDocument13 pagesOCHEM Practice FinalsNoleNo ratings yet
- Practice Sn12Document5 pagesPractice Sn12Ram KrishnaNo ratings yet
- Haloalkanes and HaloarenesDocument18 pagesHaloalkanes and HaloarenesBhavesh KNo ratings yet
- Practice E12Document10 pagesPractice E12Stephen BoandohNo ratings yet
- SC12 (1) .1 AlkanesDocument3 pagesSC12 (1) .1 AlkanesAishah AzliNo ratings yet
- MCQ Chapter 9 Haloalkanes and HaloarenesDocument2 pagesMCQ Chapter 9 Haloalkanes and HaloarenesNinaNo ratings yet
- Tutorial 2 Answers NewDocument15 pagesTutorial 2 Answers Newsangonomiya21No ratings yet
- Chapter 06Document47 pagesChapter 06AC BañaresNo ratings yet
- TS21.C12.01 Classification of Haloalkanes and Haloarenes 25-04-2021Document3 pagesTS21.C12.01 Classification of Haloalkanes and Haloarenes 25-04-2021VaradaNo ratings yet
- Practice Chapter 2 Hydrocarbon Frameworks - AlkanesDocument22 pagesPractice Chapter 2 Hydrocarbon Frameworks - AlkanesNadzirah ZaimNo ratings yet
- CHM2000 Group Work 07Document3 pagesCHM2000 Group Work 07Aleeya JulitaNo ratings yet
- Hydrocarbon (SCH Sir)Document5 pagesHydrocarbon (SCH Sir)ArthGadaNo ratings yet
- Organic Chemistry 9Th Edition Carey Test Bank Full Chapter PDFDocument35 pagesOrganic Chemistry 9Th Edition Carey Test Bank Full Chapter PDFtonya.paongo686100% (11)
- Alkenes2 CNBFDocument19 pagesAlkenes2 CNBFAditya RaiNo ratings yet
- Đề Cương Học Phần Hoá Hữu Cơ Lớp D2022Document17 pagesĐề Cương Học Phần Hoá Hữu Cơ Lớp D2022Cảnh NguyễnNo ratings yet
- Iitian'S Hub: Assignment # 1 General Organic Chemistry ChemistryDocument11 pagesIitian'S Hub: Assignment # 1 General Organic Chemistry ChemistrySAHILI RANENo ratings yet
- Tutorial 5Document4 pagesTutorial 5Eqieyn JerrNo ratings yet
- Revision 12 - IUPAC OrganicDocument9 pagesRevision 12 - IUPAC Organicnaruto.newgodNo ratings yet
- Chem. Imp. QuestionsDocument16 pagesChem. Imp. QuestionsHimanshu NikhurpaNo ratings yet
- De Cuong HHCDocument42 pagesDe Cuong HHCNguyễn DuyênNo ratings yet
- ch16 Blackman2e Answers Odd QuestionsDocument23 pagesch16 Blackman2e Answers Odd QuestionsCarlosE.NeriNo ratings yet
- Organic Ps Chapter 7Document33 pagesOrganic Ps Chapter 7Mond DamascoNo ratings yet
- Essential Organic Chemistry 2nd Edition Bruice Test BankDocument21 pagesEssential Organic Chemistry 2nd Edition Bruice Test Bankmarykirbyifsartwckp100% (15)
- CH 06 Revi4Document9 pagesCH 06 Revi4Adedire FisayoNo ratings yet
- Haloalkanes and HaloarenesDocument68 pagesHaloalkanes and Haloarenesmrpulkit20No ratings yet
- Core Organic Assessed Homework - 2Document3 pagesCore Organic Assessed Homework - 2Bintou CoulibalyNo ratings yet
- Chemguide - Answers: Fragmentation PatternsDocument2 pagesChemguide - Answers: Fragmentation PatternsKhondokar TarakkyNo ratings yet
- Chapter 5.1Document3 pagesChapter 5.1Aliff AmirudinNo ratings yet
- 12 Organic Chemistry: Some Basic Principles and Techniques: SolutionsDocument40 pages12 Organic Chemistry: Some Basic Principles and Techniques: SolutionssharmilaNo ratings yet
- MCQ Halo Alkanes and ArenesDocument27 pagesMCQ Halo Alkanes and ArenessarahNo ratings yet
- Exam Organic Chemistry I WhittenDocument19 pagesExam Organic Chemistry I WhittenDaniel Baylosis Asong60% (5)
- Organic Halogen Compounds UpdatedDocument20 pagesOrganic Halogen Compounds UpdatedDr. Dhondiba VishwanathNo ratings yet
- Cls Jeead-17-18 Xi Che Target-4 Set-2 Chapter-13Document34 pagesCls Jeead-17-18 Xi Che Target-4 Set-2 Chapter-13Jotiraj Parihar50% (2)
- 2240 Exam1 Practice Sp03Document9 pages2240 Exam1 Practice Sp03Romil PatelNo ratings yet
- Jee-Main ChemistryDocument5 pagesJee-Main ChemistryAbhishek SaravananNo ratings yet
- 1 - Classification & Nomeclature of Organic CompoundsDocument8 pages1 - Classification & Nomeclature of Organic CompoundsarvindkrishnaNo ratings yet
- Differentiation of Chiral Compounds Using NMR SpectroscopyFrom EverandDifferentiation of Chiral Compounds Using NMR SpectroscopyNo ratings yet
- Hyrdogen Storage TechnologiesFrom EverandHyrdogen Storage TechnologiesMehmet SankirNo ratings yet
- Current Evidence-Based Resources Used by Healthcare Professionals Versus PT Management For Falls ManagementDocument4 pagesCurrent Evidence-Based Resources Used by Healthcare Professionals Versus PT Management For Falls Managementabhijeet_sangwanNo ratings yet
- Chapter 1: Getting Started With Java Language: RemarksDocument1 pageChapter 1: Getting Started With Java Language: Remarksabhijeet_sangwanNo ratings yet
- NURSERY (3.5 Years To 4.5 Years) : PreschoolersDocument1 pageNURSERY (3.5 Years To 4.5 Years) : Preschoolersabhijeet_sangwanNo ratings yet
- Computer CentreDocument1 pageComputer Centreabhijeet_sangwanNo ratings yet
- Drawing Competition Entry FormDocument1 pageDrawing Competition Entry Formabhijeet_sangwan0% (2)
- Directions: Melbourne Level 1: Powered byDocument1 pageDirections: Melbourne Level 1: Powered byabhijeet_sangwanNo ratings yet
- Chapter 34: Strings 97: Examples 97Document1 pageChapter 34: Strings 97: Examples 97abhijeet_sangwanNo ratings yet
- Development and Learning in Outdoor PlayDocument1 pageDevelopment and Learning in Outdoor Playabhijeet_sangwanNo ratings yet
- Unit Testing: Javascript: The Good PartsDocument1 pageUnit Testing: Javascript: The Good Partsabhijeet_sangwanNo ratings yet
- To Age Group: The Wealthy Yeoman Wanted To Move To The CityDocument1 pageTo Age Group: The Wealthy Yeoman Wanted To Move To The Cityabhijeet_sangwanNo ratings yet
- To Age Group: Viri Des NT Vål TailDocument1 pageTo Age Group: Viri Des NT Vål Tailabhijeet_sangwanNo ratings yet
- Pages From Oreilly - Javascript.patterns - Sep.2010Document1 pagePages From Oreilly - Javascript.patterns - Sep.2010abhijeet_sangwanNo ratings yet
- Test 2Document1 pageTest 2abhijeet_sangwanNo ratings yet
- 4 Periodic Table 3Document1 page4 Periodic Table 3abhijeet_sangwanNo ratings yet
- The Bio-Medical Waste (Management and Handling) Rules, 1998: 8. AuthorisationDocument23 pagesThe Bio-Medical Waste (Management and Handling) Rules, 1998: 8. Authorisationabhijeet_sangwanNo ratings yet
- Test 1Document1 pageTest 1abhijeet_sangwanNo ratings yet
- From Fax Pages Phone Date Re CC: Urgent For Review Please Comment Please Reply Please RecycleDocument1 pageFrom Fax Pages Phone Date Re CC: Urgent For Review Please Comment Please Reply Please Recycleabhijeet_sangwanNo ratings yet
- 4 Periodic Table 1Document1 page4 Periodic Table 1abhijeet_sangwanNo ratings yet
- Module - 2: 4.1 Early AttemptsDocument1 pageModule - 2: 4.1 Early Attemptsabhijeet_sangwanNo ratings yet