Professional Documents
Culture Documents
Prop
Prop
Uploaded by
api-2968338590 ratings0% found this document useful (0 votes)
3 views1 pageWater has several important properties that allow it to serve critical functions for living organisms. It can dissolve ions and polar molecules, allowing substances to move and react. Its high specific heat capacity and heat of vaporization enable temperature regulation. As a liquid, water has higher density than ice, allowing ice to float and insulate aquatic life. Its surface tension and cohesion facilitate fluid transport in plants and allow some insects to walk on water. Water also acts as a reagent in key biochemical reactions like photosynthesis and respiration.
Original Description:
Original Title
prop
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentWater has several important properties that allow it to serve critical functions for living organisms. It can dissolve ions and polar molecules, allowing substances to move and react. Its high specific heat capacity and heat of vaporization enable temperature regulation. As a liquid, water has higher density than ice, allowing ice to float and insulate aquatic life. Its surface tension and cohesion facilitate fluid transport in plants and allow some insects to walk on water. Water also acts as a reagent in key biochemical reactions like photosynthesis and respiration.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
3 views1 pageProp
Prop
Uploaded by
api-296833859Water has several important properties that allow it to serve critical functions for living organisms. It can dissolve ions and polar molecules, allowing substances to move and react. Its high specific heat capacity and heat of vaporization enable temperature regulation. As a liquid, water has higher density than ice, allowing ice to float and insulate aquatic life. Its surface tension and cohesion facilitate fluid transport in plants and allow some insects to walk on water. Water also acts as a reagent in key biochemical reactions like photosynthesis and respiration.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
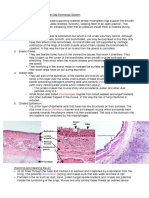
Property of Water
Functions
Water is a solvent for
ions and polar
molecules.
- Once a chemical is in a solution it is free to react with other
chemicals (metabolism).
- It pushes polar molecules together (hydrophobic interactions).
- It acts as a transport medium in the blood, lymphatic,
digestive, and excretory systems of animals and the vascular
tissues of plants.
High specific heat
capacity
- Allows temperatures within cells and bodies to be more
constant than that of the air around them.
- Biochemical reactions can occur at a more constant rate and
are less easily affected by extremes of temperature.
- Large bodies of water change temperatures more slowly than
the environment around them, providing a stable environment
for aquatic organism.
High latent heat of
vapourisation
- Organisms can use evaporation as a cooling mechanism
(sweating/transpiration) while preventing dehydration.
- Less likely for large bodies of water to freeze, so aquatic
organisms have higher chances of survival.
Liquid water has
higher density than
ice
- Ice floats on water, insulating the water underneath, so the
chances of survival for aquatic organisms increase.
- Changes in water density cause currents which maintain the
circulation of nutrients in the oceans.
High surface tension
and cohesion
- Water can move in long unbroken columns in the vascular
tissues of plants.
- Allows certain organisms to settle or skate over the waters
surface (e.g. pond skaters).
Water is a reagent
(sometimes)
- Hydrogen is used as a fuel in plants.
- Oxygen is needed for aerobic respiration.
- Water is essential for hydrolysis reactions.
You might also like
- The Biological Importance of WaterDocument2 pagesThe Biological Importance of WaterSophie Talib0% (3)
- Biological Importance of WaterDocument2 pagesBiological Importance of WaterHuyen Bui100% (1)
- Biological Importance of Water-EssayDocument2 pagesBiological Importance of Water-EssayBhavya AhujaNo ratings yet
- Water 1Document9 pagesWater 1Michael Nyaongo100% (1)
- 30 Water Page 1Document2 pages30 Water Page 1ryuzaki589100% (1)
- Is Water Really Necessary For Life? DiscussDocument3 pagesIs Water Really Necessary For Life? DiscussNejdiDarmaNo ratings yet
- Documento 2Document3 pagesDocumento 2Agustin GarciaNo ratings yet
- Chemistry, Physics and Biology of WaterDocument2 pagesChemistry, Physics and Biology of WaterJohn OsborneNo ratings yet
- Essay WaterDocument1 pageEssay WaterSimon AwberyNo ratings yet
- The ChemicalDocument2 pagesThe ChemicalVinu NishiNo ratings yet
- 1.what Is The Important of Water in Life ?Document2 pages1.what Is The Important of Water in Life ?CQChoongNo ratings yet
- Biochemistry WaterDocument3 pagesBiochemistry WaterOrane CassanovaNo ratings yet
- Introduction To Biochemistry: Group 5 Section 2Document12 pagesIntroduction To Biochemistry: Group 5 Section 2Carmina DinerosNo ratings yet
- Water Has A High Specific HeatDocument2 pagesWater Has A High Specific HeatSharee Jane DicamNo ratings yet
- A1.1 WaterDocument36 pagesA1.1 WatergabrieldelriobetancourtNo ratings yet
- PDF DocumentDocument6 pagesPDF DocumentKevin MaharajNo ratings yet
- Properties of Water: SourcesDocument6 pagesProperties of Water: SourcesRichie Juls BacalsoNo ratings yet
- Biol 325 Notes - BiochemistryDocument27 pagesBiol 325 Notes - BiochemistryKargboNo ratings yet
- WaterDocument2 pagesWaterJake ChinNo ratings yet
- Updated Biochemistry Notes 2022Document17 pagesUpdated Biochemistry Notes 2022vandytommy308No ratings yet
- Essay WaterDocument1 pageEssay WatersayraNo ratings yet
- Water: Water Molecule's StructureDocument2 pagesWater: Water Molecule's StructureCheong Yong XuanNo ratings yet
- المحاضرة ٢Document7 pagesالمحاضرة ٢Mahmoud KhlifaNo ratings yet
- Bio1TC1 1.1 WaterDocument34 pagesBio1TC1 1.1 WaterFounder ChaNo ratings yet
- Chapter 1: Biological Molecules: WaterDocument15 pagesChapter 1: Biological Molecules: WaterChong Hyen100% (1)
- Physical Properties of Water: Richie Juls Bacalso Ma. Annalyn Mulig Wendell JualoDocument15 pagesPhysical Properties of Water: Richie Juls Bacalso Ma. Annalyn Mulig Wendell JualoRichie Juls BacalsoNo ratings yet
- Biology-Biological-Molecules-Part - HTML: WaterDocument3 pagesBiology-Biological-Molecules-Part - HTML: Watersy ing ingNo ratings yet
- Biology NotesDocument161 pagesBiology NotesBlohsh KeenenNo ratings yet
- Plant Water RelationsDocument27 pagesPlant Water RelationsNam GonzalesNo ratings yet
- T1.11 Chemicals of Life-1Document3 pagesT1.11 Chemicals of Life-1KudaNo ratings yet
- 2.2 Presentation WaterDocument98 pages2.2 Presentation WaterSurya KarthikeyanNo ratings yet
- Biological Importance of WaterDocument1 pageBiological Importance of WaterAce Thor YaheeNo ratings yet
- General Chemistry 2 - Properties of WaterDocument13 pagesGeneral Chemistry 2 - Properties of Watergarcia.ohnie5101No ratings yet
- 5 Fascinating Facts About WaterDocument1 page5 Fascinating Facts About WaterFiona ZhangNo ratings yet
- Types of WaterDocument23 pagesTypes of WaterTSha MeunierNo ratings yet
- Chapter 2Document4 pagesChapter 2linhdo090703No ratings yet
- Biochemistry Laboratory Water and ElectrolytesDocument3 pagesBiochemistry Laboratory Water and ElectrolytesJed Kachel BugayongNo ratings yet
- 02 WaterDocument4 pages02 WaterSadia BatoolNo ratings yet
- Biological Molecules: WaterDocument34 pagesBiological Molecules: WaterYing Shuang100% (1)
- Unique Properties of WaterDocument4 pagesUnique Properties of WaterImran KhanNo ratings yet
- Human AnaPhysio Assignment2Document19 pagesHuman AnaPhysio Assignment2Mark Piedad MillanoNo ratings yet
- Lesson 2 Matter in The Liquid PhaseDocument27 pagesLesson 2 Matter in The Liquid PhaseDarren Daniel InfanteNo ratings yet
- WATERDocument4 pagesWATERKristine AbelladaNo ratings yet
- The Ecological Importance of Water and Its PhysicalDocument11 pagesThe Ecological Importance of Water and Its PhysicalRe HanaNo ratings yet
- Lec4 Role of Water in Food and Its Shelf LifeDocument17 pagesLec4 Role of Water in Food and Its Shelf LifeDIBINo ratings yet
- Origin and Chemical Basis of Life FInalDocument12 pagesOrigin and Chemical Basis of Life FInalrhyshadevanrayosoNo ratings yet
- BIOLOGICAL MOLECULES-waterDocument4 pagesBIOLOGICAL MOLECULES-watertsteadmanNo ratings yet
- CHAPTER 4 - Topic 4.1-Water ChemistryDocument27 pagesCHAPTER 4 - Topic 4.1-Water Chemistryasyraf azlan99No ratings yet
- Bio Lab Report Water PropertiesDocument2 pagesBio Lab Report Water Propertiesapi-439891660No ratings yet
- No Class Monday!Document48 pagesNo Class Monday!E. WatsonNo ratings yet
- EMGBS-Bio 11. U.2 NoteDocument77 pagesEMGBS-Bio 11. U.2 Notenafhire2021No ratings yet
- Water's Most Important Biochemical Role: The SolventDocument2 pagesWater's Most Important Biochemical Role: The SolventMöhä HàmouliliNo ratings yet
- Envisci ReviewerDocument4 pagesEnvisci ReviewerTremolo backNo ratings yet
- Introduction To Freshwater Ecology: Key Words: Ecosystem, Food Web, Lakes, RiversDocument17 pagesIntroduction To Freshwater Ecology: Key Words: Ecosystem, Food Web, Lakes, RiverssheriefmuhammedNo ratings yet
- How Plant and Animal Adapt To Aquatic HabitatDocument16 pagesHow Plant and Animal Adapt To Aquatic HabitatCharles Amaechi100% (2)
- Revision Sheet - WaterDocument1 pageRevision Sheet - WaterannafiiNo ratings yet
- Activity Sheet 4 Properties of WaterDocument14 pagesActivity Sheet 4 Properties of Waterhumptydumpty.elleNo ratings yet
- Chem101 Ho8Document17 pagesChem101 Ho8Claire TaborNo ratings yet
- Biochemistry WaterDocument3 pagesBiochemistry WaterRory DrewNo ratings yet
- Negative Effects of Growing Herbicide Resistant Crops IncludeDocument3 pagesNegative Effects of Growing Herbicide Resistant Crops Includeapi-296833859No ratings yet
- TranscriptoneDocument2 pagesTranscriptoneapi-296833859No ratings yet
- Photorespiration: Bundle Sheath CellsDocument1 pagePhotorespiration: Bundle Sheath Cellsapi-296833859No ratings yet
- Absorbance Spectrum Action SpectrumDocument2 pagesAbsorbance Spectrum Action Spectrumapi-296833859No ratings yet
- Selective Advantage Natural Selection Selection Pressure: Speciation SpeciesDocument2 pagesSelective Advantage Natural Selection Selection Pressure: Speciation Speciesapi-296833859No ratings yet
- Inbreeding Interbreeding Outbreeding Inbreeding DepressionDocument1 pageInbreeding Interbreeding Outbreeding Inbreeding Depressionapi-296833859No ratings yet
- Background Genes Progeny TestingDocument1 pageBackground Genes Progeny Testingapi-296833859No ratings yet
- Selection PressuresDocument1 pageSelection Pressuresapi-296833859No ratings yet
- The Founder Effect: Genetic DriftDocument1 pageThe Founder Effect: Genetic Driftapi-296833859No ratings yet
- Selective AdvantagesDocument2 pagesSelective Advantagesapi-296833859No ratings yet
- Coevolution Fitness Natural Selection: Discontinuous VariationDocument2 pagesCoevolution Fitness Natural Selection: Discontinuous Variationapi-296833859No ratings yet
- Energy DensityDocument1 pageEnergy Densityapi-296833859No ratings yet
- Photophosphorylation:: PhotoactivationDocument1 pagePhotophosphorylation:: Photoactivationapi-296833859No ratings yet
- PhotolysisDocument1 pagePhotolysisapi-296833859No ratings yet
- Limiting FactorDocument2 pagesLimiting Factorapi-296833859No ratings yet
- Anaerobic Respiration (In The: CytoplasmDocument1 pageAnaerobic Respiration (In The: Cytoplasmapi-296833859No ratings yet
- GahhDocument1 pageGahhapi-296833859No ratings yet
- High Energy Bonds: Universal Intermediary MoleculeDocument2 pagesHigh Energy Bonds: Universal Intermediary Moleculeapi-296833859No ratings yet
- Overcrowded Substandard Housing Low ImmunityDocument2 pagesOvercrowded Substandard Housing Low Immunityapi-296833859No ratings yet
- Cytoplasm: GlycolysisDocument4 pagesCytoplasm: Glycolysisapi-296833859No ratings yet
- Organic Molecule Respiration Potential Energy Chemical Potential EnergyDocument1 pageOrganic Molecule Respiration Potential Energy Chemical Potential Energyapi-296833859No ratings yet
- AntibioticsDocument2 pagesAntibioticsapi-296833859No ratings yet
- Glycoproteins Called Mucin: DesiccationDocument2 pagesGlycoproteins Called Mucin: Desiccationapi-296833859No ratings yet
- CholeraDocument1 pageCholeraapi-296833859No ratings yet
- Cardiac Cycle: PacemakerDocument3 pagesCardiac Cycle: Pacemakerapi-296833859No ratings yet
- Passive Smoking: CarcinogensDocument3 pagesPassive Smoking: Carcinogensapi-296833859No ratings yet
- Cardiac Muscle: MyogenicDocument2 pagesCardiac Muscle: Myogenicapi-296833859No ratings yet
- Gas Exchange: One (1.8 CM) Yes Yes Yes Yes No YesDocument2 pagesGas Exchange: One (1.8 CM) Yes Yes Yes Yes No Yesapi-296833859No ratings yet