Professional Documents
Culture Documents
Cell Walls and The Extracellular Matrix - The Cell - Ncbi Bookshelf
Cell Walls and The Extracellular Matrix - The Cell - Ncbi Bookshelf
Uploaded by
api-302662836Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cell Walls and The Extracellular Matrix - The Cell - Ncbi Bookshelf
Cell Walls and The Extracellular Matrix - The Cell - Ncbi Bookshelf
Uploaded by
api-302662836Copyright:
Available Formats
11/17/2015
CellWallsandtheExtracellularMatrixTheCellNCBIBookshelf
NCBIBookshelf.AserviceoftheNationalLibraryofMedicine,NationalInstitutesofHealth.
CooperGM.TheCell:AMolecularApproach.2ndedition.Sunderland(MA):SinauerAssociates2000.
CellWallsandtheExtracellularMatrix
Althoughcellboundariesaredefinedbytheplasmamembrane,manycellsaresurroundedbyan
insolublearrayofsecretedmacromolecules.Cellsofbacteria,fungi,algae,andhigherplantsare
surroundedbyrigidcellwalls,whichareanintegralpartofthecell.Althoughnotencasedincell
walls,animalcellsintissuesarecloselyassociatedwithanextracellularmatrixcomposedof
proteinsandpolysaccharides.Theextracellularmatrixnotonlyprovidesstructuralsupporttocells
andtissues,butalsoplaysimportantrolesinregulatingthebehaviorofcellsinmulticellular
organisms.
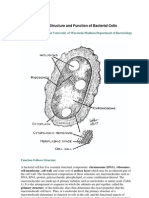
BacterialCellWalls
Therigidcellwallsofbacteriadeterminecellshapeandpreventthecellfromburstingasaresultof
osmoticpressure.Thestructureoftheircellwallsdividesbacteriaintotwobroadclassesthatcan
bedistinguishedbyastainingprocedureknownastheGramstain,developedbyChristianGramin
1884(Figure12.44).Asdescribedearlierinthischapter,Gramnegativebacteria(suchasE.coli)
haveadualmembranesystem,inwhichtheplasmamembraneissurroundedbyapermeableouter
membrane.Thesebacteriahavethincellwallslocatedbetweentheirinnerandoutermembranes.In
contrast,Grampositivebacteria(suchasthecommonhumanpathogenStaphylococcusaureus)
haveonlyasingleplasmamembrane,whichissurroundedbyamuchthickercellwall.
Figure12.44
Bacterialcellwalls.TheplasmamembraneofGramnegative
bacteriaissurroundedbyathincellwallbeneaththeouter
membrane.Grampositivebacterialackoutermembranesand
havethickcellwalls.
Despitethesestructuraldifferences,theprincipalcomponentofthecellwallsofbothGram
positiveandGramnegativebacteriaisapeptidoglycan(Figure12.45)consistingoflinear
polysaccharidechainscrosslinkedbyshortpeptides.Becauseofthiscrosslinkedstructure,the
peptidoglycanformsastrongcovalentshellaroundtheentirebacterialcell.Interestingly,the
uniquestructureoftheircellwallsalsomakesbacteriavulnerabletosomeantibiotics.Penicillin,for
example,inhibitstheenzymeresponsibleforformingcrosslinksbetweendifferentstrandsofthe
peptidoglycan,therebyinterferingwithcellwallsynthesisandblockingbacterialgrowth.
Figure12.45
ThepeptidoglycanofE.coli.Polysaccharidechainsconsistof
alternatingNacetylglucosamine(NAG)andNacetylmuramic
acid(NAM)residuesjoinedby(14)glycosidicbonds.
Parallelchainsarecrosslinkedbytetrapeptidesattachedto
(more...)
PlantCellWalls
Incontrasttobacteria,thecellwallsofeukaryotes(includingfungi,algae,andhigherplants)are
http://www.ncbi.nlm.nih.gov/books/NBK9874/
1/9
11/17/2015
CellWallsandtheExtracellularMatrixTheCellNCBIBookshelf
composedprincipallyofpolysaccharides(Figure12.46).Thebasicstructuralpolysaccharideof
fungalcellwallsischitin(apolymerofNacetylglucosamineresidues),whichalsoformsthe
exoskeletonofarthropods(e.g.,theshellsofcrabs).Thecellwallsofmostalgaeandhigherplants
arecomposedprincipallyofcellulose,whichisthesinglemostabundantpolymeronEarth.
Celluloseisalinearpolymerofglucoseresidues,oftencontainingmorethan10,000glucose
monomers.Theglucoseresiduesarejoinedby(14)linkages,whichallowthepolysaccharide
toformlongstraightchains.Severaldozensuchchainsthenassociateinparallelwithoneanother
toformcellulosemicrofibrils,whichcanextendformanymicrometersinlength.
Figure12.46
Polysaccharidesoffungalandplantcellwalls.(A)Chitin(the
principalpolysaccharideoffungalcellwalls)isalinear
polymerofNacetylglucosamineresidues,whereascelluloseis
alinearpolymerofglucose.Thecarbohydratemonomersare
joined(more...)
Withinthecellwall,cellulosemicrofibrilsareembeddedinamatrixconsistingofproteinsandtwo
othertypesofpolysaccharides:hemicellulosesandpectins(Figure12.47).Hemicellulosesare
highlybranchedpolysaccharidesthatarehydrogenbondedtothesurfaceofcellulosemicrofibrils.
Thiscrosslinksthecellulosemicrofibrilsintoanetworkoftough,fibrousmolecules,whichis
responsibleforthemechanicalstrengthofplantcellwalls.Pectinsarebranchedpolysaccharides
containingalargenumberofnegativelychargedgalacturonicacidresidues.Becauseofthese
multiplenegativecharges,pectinsbindpositivelychargedions(suchasCa2+)andtrapwater
moleculestoformgels.Anillustrationoftheirgelformingpropertiesisprovidedbythefactthat
jamsandjelliesareproducedbytheadditionofpectinstofruitjuice.Inthecellwall,thepectins
formagellikenetworkthatisinterlockedwiththecrosslinkedcellulosemicrofibrils.Inaddition,
cellwallscontainavarietyofglycoproteinsthatareincorporatedintothematrixandarethoughtto
providefurtherstructuralsupport.
Figure12.47
Modelofaplantcellwall.(A)Structuresofarepresentative
hemicellulose(xyloglucan)andpectin(rhamnogalacturonan).
Xyloglucanconsistsofabackboneofglucose(Glc)residues
withsidechainsofxylose(Xyl),galactose(Gal),andfucose
(Fuc).(more...)
Boththestructureandfunctionofcellwallschangeasplantcellsdevelop.Thewallsofgrowing
plantcells(calledprimarycellwalls)arerelativelythinandflexible,allowingthecelltoexpandin
size.Oncecellshaveceasedgrowth,theyfrequentlylaydownsecondarycellwallsbetweenthe
plasmamembraneandtheprimarycellwall(Figure12.48).Suchsecondarycellwalls,whichare
http://www.ncbi.nlm.nih.gov/books/NBK9874/
2/9
11/17/2015
CellWallsandtheExtracellularMatrixTheCellNCBIBookshelf
boththickerandmorerigidthanprimarywalls,areparticularlyimportantincelltypesresponsible
forconductingwaterandprovidingmechanicalstrengthtotheplant.
Figure12.48
Primaryandsecondarycellwalls.Secondarycellwallsarelaid
downbetweentheprimarycellwallandtheplasma
membrane.Secondarywallsfrequentlyconsistofthreelayers,
whichdifferintheorientationoftheircellulosemicrofibrils.
Electronmicrographs(more...)
Primaryandsecondarycellwallsdifferincompositionaswellasinthickness.Primarycellwalls
containapproximatelyequalamountsofcellulose,hemicelluloses,andpectins.Incontrast,the
morerigidsecondarywallsgenerallylackpectinandcontain50to80%cellulose.Manysecondary
wallsarefurtherstrengthenedbylignin,acomplexpolymerofphenolicresiduesthatisresponsible
formuchofthestrengthanddensityofwood.Theorientationofcellulosemicrofibrilsalsodiffers
inprimaryandsecondarycellwalls.Thecellulosefibersofprimarywallsappeartoberandomly
arranged,whereasthoseofsecondarywallsarehighlyordered(seeFigure12.48).Secondarywalls
arefrequentlylaiddowninlayersinwhichthecellulosefibersdifferinorientation,forminga
laminatedstructurethatgreatlyincreasescellwallstrength.
Oneofthecriticalfunctionsofplantcellwallsistopreventcellswellingasaresultofosmotic
pressure.Incontrasttoanimalcells,plantcellsdonotmaintainanosmoticbalancebetweentheir
cytosolandextracellularfluids.Consequently,osmoticpressurecontinuallydrivestheflowof
waterintothecell.Thiswaterinfluxistoleratedbyplantcellsbecausetheirrigidcellwallsprevent
swellingandbursting.Instead,aninternalhydrostaticpressure(calledturgorpressure)buildsup
withinthecell,eventuallyequalizingtheosmoticpressureandpreventingthefurtherinfluxof
water.
Turgorpressureisresponsibleformuchoftherigidityofplanttissues,asisreadilyapparentfrom
examinationofadehydrated,wiltedplant.Inaddition,turgorpressureprovidesthebasisforaform
ofcellgrowththatisuniquetoplants.Inparticular,plantcellsfrequentlyexpandbytakingup
waterwithoutsynthesizingnewcytoplasmiccomponents(Figure12.49).Cellexpansionbythis
mechanismissignaledbyplanthormones(auxins)thatweakenaregionofthecellwall,allowing
turgorpressuretodrivetheexpansionofthecellinthatdirection.Asthisoccurs,thewaterthat
flowsintothecellaccumulateswithinalargecentralvacuole,sothecellexpandswithout
increasingthevolumeofitscytosol.Suchexpansioncanresultina10to100foldincreaseinthe
sizeofplantcellsduringdevelopment.
Figure12.49
Expansionofplantcells.Turgorpressuredrivestheexpansion
ofplantcellsbytheuptakeofwater,whichisaccumulatedina
largecentralvacuole.
Ascellsexpand,newcomponentsofthecellwallaredepositedoutsidetheplasmamembrane.
Matrixcomponents,includinghemicellulosesandpectins,aresynthesizedintheGolgiapparatus
andsecreted.Cellulose,however,issynthesizedbyaplasmamembraneenzymecomplex
(cellulosesynthase).Inexpandingcells,thenewlysynthesizedcellulosemicrofibrilsaredeposited
http://www.ncbi.nlm.nih.gov/books/NBK9874/
3/9
11/17/2015
CellWallsandtheExtracellularMatrixTheCellNCBIBookshelf
atrightanglestothedirectionofcellelongationanorientationthatisthoughttoplayanimportant
roleindeterminingthedirectionoffurthercellexpansion(Figure12.50).Interestingly,the
cellulosemicrofibrilsinelongatingcellwallsarelaiddowninparalleltocorticalmicrotubules
underlyingtheplasmamembrane.Thesemicrotubulesappeartodefinetheorientationofnewly
synthesizedcellulosemicrofibrils,possiblybydeterminingthedirectionofmovementofthe
cellulosesynthasecomplexesinthemembrane.Thecorticalmicrotubulesthusdefinethedirection
ofcellwallgrowth,whichinturndeterminesthedirectionofcellexpansionandultimatelythe
shapeoftheentireplant.
Figure12.50
Cellulosesynthesisduringcellelongation.Newcellulose
microfibrils,synthesizedbyaplasmamembraneenzyme
complex(cellulosesynthase),arelaiddownatrightanglesto
thedirectionofcellelongation.Thedirectionofcellulose
synthesisisparallel(more...)
TheExtracellularMatrix
Althoughanimalcellsarenotsurroundedbycellwalls,manyofthecellsintissuesofmulticellular
organismsareembeddedinanextracellularmatrixconsistingofsecretedproteinsand
polysaccharides.Theextracellularmatrixfillsthespacesbetweencellsandbindscellsandtissues
together.Onetypeofextracellularmatrixisexemplifiedbythethin,sheetlikebasallaminae,or
basementmembranes,uponwhichlayersofepithelialcellsrest(Figure12.51).Inadditionto
supportingsheetsofepithelialcells,basallaminaesurroundmusclecells,adiposecells,and
peripheralnerves.Extracellularmatrix,however,ismostabundantinconnectivetissues.For
example,thelooseconnectivetissuebeneathepithelialcelllayersconsistspredominantlyofan
extracellularmatrixinwhichfibroblastsaredistributed.Othertypesofconnectivetissue,suchas
bone,tendon,andcartilage,similarlyconsistlargelyofextracellularmatrix,whichisprincipally
responsiblefortheirstructureandfunction.
Figure12.51
Examplesofextracellularmatrix.Sheetsofepithelialcellsrest
onathinlayerofextracellularmatrixcalledabasallamina.
Beneaththebasallaminaislooseconnectivetissue,which
consistslargelyofextracellularmatrixsecretedbyfibroblasts.
(more...)
Extracellularmatricesarecomposedoftoughfibrousproteinsembeddedinagellike
polysaccharidegroundsubstanceadesignbasicallysimilartothatofplantcellwalls.Inaddition
tofibrousstructuralproteinsandpolysaccharides,theextracellularmatrixcontainsadhesion
proteinsthatlinkcomponentsofthematrixbothtooneanotherandtoattachedcells.The
differencesbetweenthevarioustypesofextracellularmatrixresultfromvariationsonthisgeneral
theme.Forexample,tendonscontainahighproportionoffibrousproteins,whereascartilage
containsahighconcentrationofpolysaccharidesthatformafirmcompressionresistantgel.In
bone,theextracellularmatrixishardenedbydepositionofcalciumphosphatecrystals.The
http://www.ncbi.nlm.nih.gov/books/NBK9874/
4/9
11/17/2015
CellWallsandtheExtracellularMatrixTheCellNCBIBookshelf
sheetlikestructureofbasallaminaealsoresultsfromtheutilizationofmatrixcomponentsthatdiffer
fromthosefoundinconnectivetissues.
Themajorstructuralproteinoftheextracellularmatrixiscollagen,whichisthesinglemost
abundantproteininanimaltissues.Thecollagensarealargefamilyofproteins,containingatleast
19differentmembers.Theyarecharacterizedbytheformationoftriplehelicesinwhichthree
polypeptidechainsarewoundtightlyaroundoneanotherinaropelikestructure(Figure12.52).
ThetriplehelixdomainsofthecollagensconsistofrepeatsoftheaminoacidsequenceGlyXY.
Aglycine(thesmallestaminoacid,withasidechainconsistingonlyofahydrogen)isrequiredin
everythirdpositioninorderforthepolypeptidechainstopacktogethercloseenoughtoformthe
collagentriplehelix.ProlineisfrequentlyfoundintheXpositionandhydroxyprolineintheY
positionbecauseoftheirringstructure,theseaminoacidsstabilizethehelicalconformationsofthe
polypeptidechains.Theunusualaminoacidhydroxyprolineisformedwithintheendoplasmic
reticulumbymodificationofprolineresiduesthathavealreadybeenincorporatedintocollagen
polypeptidechains(Figure12.53).Lysineresiduesincollagenarealsofrequentlyconvertedto
hydroxylysines.Thehydroxylgroupsofthesemodifiedaminoacidsarethoughttostabilizethe
collagentriplehelixbyforminghydrogenbondsbetweenpolypeptidechains.Theseaminoacids
arerarelyfoundinotherproteins,althoughhydroxyprolineisalsocommoninsomeofthe
glycoproteinsofplantcellwalls.
Figure12.52
Structureofcollagen.(A)Threepolypeptidechainscoil
aroundoneanotherinacharacteristictriplehelixstructure.(B)
Theaminoacidsequenceofacollagentriplehelixdomain
consistsofGlyXYrepeats,inwhichXisfrequentlyproline
andYis(more...)
Figure12.53
Formationofhydroxyproline.Prolylhydroxylaseconverts
prolineresiduesincollagentohydroxyproline.
Themostabundanttypeofcollagen(typeIcollagen)isoneofthefibrilformingcollagensthatare
thebasicstructuralcomponentsofconnectivetissues(Table12.2).Thepolypeptidechainsofthese
collagensconsistofapproximatelyathousandaminoacidsor330GlyXYrepeats.Afterbeing
secretedfromthecell,thesecollagensassembleintocollagenfibrilsinwhichthetriplehelical
moleculesareassociatedinregularstaggeredarrays(Figure12.54).Thesefibrilsdonotform
withinthecell,becausethefibrilformingcollagensaresynthesizedassolubleprecursors
(procollagens)thatcontainnonhelicalsegmentsatbothendsofthepolypeptidechain.Procollagen
iscleavedtocollagenafteritssecretion,sotheassemblyofcollagenintofibrilstakesplaceonly
outsidethecell.Theassociationofcollagenmoleculesinfibrilsisfurtherstrengthenedbythe
formationofcovalentcrosslinksbetweenthesidechainsoflysineandhydroxylysineresidues.
http://www.ncbi.nlm.nih.gov/books/NBK9874/
5/9
11/17/2015
CellWallsandtheExtracellularMatrixTheCellNCBIBookshelf
Frequently,thefibrilsfurtherassociatewithoneanothertoformcollagenfibers,whichcanbe
severalmicrometersindiameter.
Table12.2
RepresentativeMembersoftheCollagenFamily.
Figure12.54
Collagenfibrils.(A)Collagenmoleculesassembleinaregular
staggeredarraytoformfibrils.Themoleculesoverlapbyone
fourthoftheirlength,andthereisashortgapbetweentheN
terminusofonemoleculeandtheCterminusofthenext.The
assembly(more...)
Severalothertypesofcollagendonotformfibrilsbutplaydistinctrolesinvariouskindsof
extracellularmatrices.Inadditiontothefibrilformingcollagens,connectivetissuescontainfibril
associatedcollagens,whichbindtothesurfaceofcollagenfibrilsandlinkthembothtooneanother
andtoothermatrixcomponents.Basallaminaeformfromadifferenttypeofcollagen(typeIV
collagen),whichisanetworkformingcollagen(Figure12.55).TheGlyXYrepeatsofthese
collagensarefrequentlyinterruptedbyshortnonhelicalsequences.Becauseoftheseinterruptions,
thenetworkformingcollagensaremoreflexiblethanthefibrilformingcollagens.Consequently,
theyassembleintotwodimensionalcrosslinkednetworksinsteadoffibrils.Yetanothertypeof
collagenformsanchoringfibrils,whichlinksomebasallaminaetounderlyingconnectivetissues.
Figure12.55
TypeIVcollagen.(A)TheGlyXYrepeatstructureoftype
IVcollagen(yellow)isinterruptedbymultiplenonhelical
sequences(bars).(B)ElectronmicrographofatypeIV
collagennetwork.(B,P.D.YurchencoandJ.C.Schittny,
1990.FASEBJ.4:1577.)(more...)
Connectivetissuesalsocontainelasticfibers,whichareparticularlyabundantinorgansthat
regularlystretchandthenreturntotheiroriginalshape.Thelungs,forexample,stretcheachtimea
breathisinhaledandreturntotheiroriginalshapewitheachexhalation.Elasticfibersare
composedprincipallyofaproteincalledelastin,whichiscrosslinkedintoanetworkbycovalent
bondsformedbetweenthesidechainsoflysineresidues(similartothosefoundincollagen).This
networkofcrosslinkedelastinchainsbehaveslikearubberband,stretchingundertensionandthen
snappingbackwhenthetensionisreleased.
Thefibrousstructuralproteinsoftheextracellularmatrixareembeddedingelsformedfrom
polysaccharidescalledglycosaminoglycans,orGAGs,whichconsistofrepeatingunitsof
disaccharides(Figure12.56).OnesugarofthedisaccharideiseitherNacetylglucosamineorN
acetylgalactosamineandthesecondisusuallyacidic(eitherglucuronicacidoriduronicacid).With
theexceptionofhyaluronan,thesesugarsaremodifiedbytheadditionofsulfategroups.
Consequently,GAGsarehighlynegativelycharged.Likethepectinsofplantcellwalls,theybind
positivelychargedionsandtrapwatermoleculestoformhydratedgels,therebyproviding
mechanicalsupporttotheextracellularmatrix.
http://www.ncbi.nlm.nih.gov/books/NBK9874/
6/9
11/17/2015
CellWallsandtheExtracellularMatrixTheCellNCBIBookshelf
Figure12.56
Majortypesofglycosaminoglycans.Glycosaminoglycansconsistofrepeating
disaccharideunits.Withtheexceptionofhyaluronan,thesugarsfrequently
containsulfate.Heparansulfateissimilartoheparinexceptthatitcontainsfewer
sulfategroups.(more...)
HyaluronanistheonlyGAGthatoccursasasinglelongpolysaccharidechain.Alloftheother
GAGsarelinkedtoproteinstoformproteoglycans,whichcanconsistofupto95%polysaccharide
byweight.ProteoglycanscancontainasfewasoneorasmanyasmorethanahundredGAG
chainsattachedtoserineresiduesofacoreprotein.Avarietyofcoreproteins(rangingfrom10to
>500kd)havebeenidentified,sotheproteoglycansareadiversegroupofmacromolecules.In
additiontobeingcomponentsoftheextracellularmatrix,someproteoglycansarecellsurface
proteinsthatfunctionincelladhesion.
Anumberofproteoglycansinteractwithhyaluronantoformlargecomplexesintheextracellular
matrix.Awellcharacterizedexampleisaggrecan,themajorproteoglycanofcartilage(Figure
12.57).Morethanahundredchainsofchondroitinsulfateareattachedtoacoreproteinofabout
250kd,formingaproteoglycanofabout3000kd.Multipleaggrecanmoleculesthenassociatewith
chainsofhyaluronan,forminglargeaggregates(>100,000kd)thatbecometrappedinthecollagen
network.Proteoglycansalsointeractwithbothcollagenandothermatrixproteinstoformgellike
networksinwhichthefibrousstructuralproteinsoftheextracellularmatrixareembedded.For
example,perlecan(themajorheparansulfateproteoglycanofbasallaminae)bindstobothtypeIV
collagenandtotheadhesionproteinlaminin,whichisdiscussedshortly.
Figure12.57
Complexesofaggrecanandhyaluronan.Aggrecanisalarge
proteoglycanconsistingofmorethan100chondroitinsulfate
chainsjoinedtoacoreprotein.Multipleaggrecanmolecules
bindtolongchainsofhyaluronan,forminglargecomplexesin
theextracellular(more...)
Adhesionproteins,thethirdclassofextracellularmatrixconstituents,areresponsibleforlinkingthe
componentsofthematrixbothtooneanotherandtothesurfacesofcells.Theprototypeofthese
moleculesisfibronectin,whichistheprincipaladhesionproteinofconnectivetissues.Fibronectin
isadimericglycoproteinconsistingoftwopolypeptidechains,eachcontainingnearly2500amino
acids(Figure12.58).Intheextracellularmatrix,fibronectinisfurthercrosslinkedintofibrilsby
disulfidebonds.FibronectinhasbindingsitesforbothcollagenandGAGs,soitcrosslinksthese
matrixcomponents.Adistinctsiteonthefibronectinmoleculeisrecognizedbycellsurface
receptorsandisthusresponsiblefortheattachmentofcellstotheextracellularmatrix.
Figure12.58
Structureoffibronectin.Fibronectinisadimerofsimilar
polypeptidechainsjoinedbydisulfidebondsneartheC
terminus.Sitesforbindingtoproteoglycans,cells,and
http://www.ncbi.nlm.nih.gov/books/NBK9874/
7/9
11/17/2015
CellWallsandtheExtracellularMatrixTheCellNCBIBookshelf
collagenareindicated.Themoleculealsocontainsadditional
bindingsitesthat(more...)
Basallaminaecontainadistinctadhesionproteincalledlaminin(Figure12.59).LiketypeIV
collagen,lamininscanselfassembleintomeshlikepolymers.Suchlamininnetworksarethemajor
structuralcomponentsofthebasallaminaesynthesizedinveryearlyembryos,whichdonot
containcollagen.Thelamininsalsohavebindingsitesforcellsurfacereceptors,typeIVcollagen,
andperlecan.Inaddition,lamininsaretightlyassociatedwithanotheradhesionprotein,called
entactinornidogen,whichalsobindstotypeIVcollagen.Asaresultofthesemultiple
interactions,laminin,entactin,typeIVcollagen,andperlecanformcrosslinkednetworksinthe
basallamina.
Figure12.59
Structureoflaminin.Lamininconsistsofthreepolypeptide
chainsdesignatedA,B1,andB2.Someofthebindingsitesfor
entactin,typeIVcollagen,proteoglycans,andcellsurface
receptorsareindicated.
Themajorcellsurfacereceptorsresponsiblefortheattachmentofcellstotheextracellularmatrix
aretheintegrins.Theintegrinsareafamilyoftransmembraneproteinsconsistingoftwosubunits,
designatedand(Figure12.60).Morethan20differentintegrins,formedfromcombinationsof
18knownsubunitsand8knownsubunits,havebeenidentified.Theintegrinsbindtoshort
aminoacidsequencespresentinmultiplecomponentsoftheextracellularmatrix,including
collagen,fibronectin,andlaminin.Thefirstsuchintegrinbindingsitetobecharacterizedwasthe
sequenceArgGlyAsp,whichisrecognizedbyseveralmembersoftheintegrinfamily.Other
integrins,however,bindtodistinctpeptidesequences,includingrecognitionsequencesin
collagensandlaminin.Transmembraneproteoglycansonthesurfaceofavarietyofcellsalsobind
tocomponentsoftheextracellularmatrixandmodulatecellmatrixinteractions.
Figure12.60
Structureofintegrins.Theintegrinsareheterodimersoftwo
transmembranesubunits,designatedand.Thesubunit
bindsdivalentcations(M2+).Thematrixbindingregionis
composedofportionsofbothsubunits.
Inadditiontoattachingcellstotheextracellularmatrix,theintegrinsserveasanchorsforthe
cytoskeleton(Figure12.61).Theresultinglinkageofthecytoskeletontotheextracellularmatrixis
responsibleforthestabilityofcellmatrixjunctions.Distinctinteractionsbetweenintegrinsandthe
cytoskeletonarefoundattwotypesofcellmatrixjunctions,focaladhesionsandhemidesmosomes,
whichwerediscussedinChapter11.Focaladhesionsattachavarietyofcells,including
fibroblasts,totheextracellularmatrix.Thecytoplasmicdomainsofthesubunitsofintegrinsat
thesecellmatrixjunctionsanchortheactincytoskeletonbyassociatingwithbundlesofactin
http://www.ncbi.nlm.nih.gov/books/NBK9874/
8/9
11/17/2015
CellWallsandtheExtracellularMatrixTheCellNCBIBookshelf
filaments.Hemidesmosomesarespecializedsitesofepithelialcellattachmentatwhichaspecific
integrin(designated64)interactswithintermediatefilamentsinsteadofwithactin.The64
integrinbindstolaminin,sohemidesmosomesanchorepithelialcellstothebasallamina.
Figure12.61
Junctionsbetweencellsandtheextracellularmatrix.Integrins
mediatetwotypesofstablejunctionsinwhichthecytoskeleton
islinkedtotheextracellularmatrix.Infocaladhesions,bundles
ofactinfilamentsareanchoredtothesubunits(more...)
Byagreementwiththepublisher,thisbookisaccessiblebythesearchfeature,butcannotbebrowsed.
Copyright2000,GeoffreyMCooper.
BookshelfID:NBK9874
http://www.ncbi.nlm.nih.gov/books/NBK9874/
9/9
You might also like
- BMI1014: Chapter 2 - Bacteria-Morphology and ClassificationDocument6 pagesBMI1014: Chapter 2 - Bacteria-Morphology and ClassificationAkmal Adib FadzilNo ratings yet
- BIO 361 - SBU Fall 2013 - Exam 1, Lectures 1 - 13Document40 pagesBIO 361 - SBU Fall 2013 - Exam 1, Lectures 1 - 13Nerdy Notes Inc.100% (1)
- Carpet ManufacturingDocument116 pagesCarpet ManufacturingShivam ShuklaNo ratings yet
- Chapter 3Document24 pagesChapter 3Dawlat Slama0% (1)
- CH 08Document11 pagesCH 08Enjie ElrassiNo ratings yet
- 2Document2 pages2Mohamad YasinNo ratings yet
- Cell Wall StructureDocument2 pagesCell Wall StructureDl Al-azizNo ratings yet
- Work Sheet HomeworkDocument4 pagesWork Sheet Homeworksherry Gideon'sNo ratings yet
- Cell Walls: Structure, Biogenesis, and ExpansionDocument26 pagesCell Walls: Structure, Biogenesis, and ExpansionRavindraNo ratings yet
- Bacterial Cell WallsDocument3 pagesBacterial Cell WallsAdel Mohsen MohamedNo ratings yet
- BCH 4107 - Plant BiochemistryDocument13 pagesBCH 4107 - Plant BiochemistryOLUWASEGUN K AfolabiNo ratings yet
- Cell The Unit of Life Class 11 Notes Biology Chapter 8-MinDocument25 pagesCell The Unit of Life Class 11 Notes Biology Chapter 8-MinAli WarisNo ratings yet
- Cover PageDocument7 pagesCover PageKamran AhmadNo ratings yet
- Cell Wall: Presented by M. Vijaya LakshmiDocument9 pagesCell Wall: Presented by M. Vijaya LakshmiATCHUNALA SAINo ratings yet
- Adhesion y Migración Celular 2021-1 - CompressedDocument44 pagesAdhesion y Migración Celular 2021-1 - CompressedNiyuhNo ratings yet
- Prokaryotic and Eukaryotic CellsDocument122 pagesProkaryotic and Eukaryotic CellsAdithya NanuvalaNo ratings yet
- Fabrizio Gelain - Novel Opportunities and Challenges Offered by Nanobiomaterials in Tissue EngineeringDocument10 pagesFabrizio Gelain - Novel Opportunities and Challenges Offered by Nanobiomaterials in Tissue EngineeringHutsDMNo ratings yet
- Traffic Light Topic 2 CellsDocument3 pagesTraffic Light Topic 2 CellsAlison HillNo ratings yet
- What Determines Cell SizeDocument22 pagesWhat Determines Cell SizefakeempireNo ratings yet
- MSC Botnay Pre Pap1 bl1 PDFDocument74 pagesMSC Botnay Pre Pap1 bl1 PDFShahid pinNo ratings yet
- Mod 2Document85 pagesMod 2BikashGuptaNo ratings yet
- Pared Celular de PlantasDocument4 pagesPared Celular de PlantasIvan Jason SalyanoNo ratings yet
- Presentation 2Document54 pagesPresentation 2hunter zoneNo ratings yet
- Bacterial Notes 2022Document5 pagesBacterial Notes 2022Banji MaikaNo ratings yet
- BSci 102, Lesson 3.2Document5 pagesBSci 102, Lesson 3.2Jays MartinezNo ratings yet
- 9 Antibacterial ReviewDocument22 pages9 Antibacterial ReviewBrahmanand KuraneNo ratings yet
- Mod 2Document24 pagesMod 2The GreatNo ratings yet
- Plant Cytoskeleton PDFDocument7 pagesPlant Cytoskeleton PDFmanoj_rkl_07No ratings yet
- Mammalian Histology AssignmentDocument9 pagesMammalian Histology AssignmentSana Sultana100% (1)
- Biyoloji Ödev Yazısı KaynakDocument4 pagesBiyoloji Ödev Yazısı KaynakbirlikteyizNo ratings yet
- Bacterial Classification, Structure, and Replication - MurrrayDocument34 pagesBacterial Classification, Structure, and Replication - MurrraysebastianNo ratings yet
- Cell Wall - WikipediaDocument75 pagesCell Wall - WikipediaSamuel AdedejiNo ratings yet
- Plant Cell WallsDocument18 pagesPlant Cell WallsAhmed OmarNo ratings yet
- General Microbiology (Chapter 3)Document18 pagesGeneral Microbiology (Chapter 3)Ashraf OsmanNo ratings yet
- Bacterial Morphology and Cell StructureDocument14 pagesBacterial Morphology and Cell StructureJe KirsteneNo ratings yet
- Cell and Molecular BiologyDocument8 pagesCell and Molecular BiologyNadine BacalangcoNo ratings yet
- 1 s2.0 S0171933523000602 MainDocument2 pages1 s2.0 S0171933523000602 Maincarolinazr24No ratings yet
- Bacterial Cell StructureDocument10 pagesBacterial Cell Structureمنتظر علي حسين A2No ratings yet
- Art Z Obrazkami1Document6 pagesArt Z Obrazkami1Rafał StanulaNo ratings yet
- Pcab 152Document7 pagesPcab 152Kevin DessiallaNo ratings yet
- Biology CH 8 BDocument25 pagesBiology CH 8 BPakhi GoelNo ratings yet
- Cell and Cell OrganellesDocument53 pagesCell and Cell OrganellesShiffa SaheedNo ratings yet
- Meningitidis), or More Than Once To Produce A Chain (Streptococcus Pyogenes), Divides Regularly in Two Planes at RightDocument14 pagesMeningitidis), or More Than Once To Produce A Chain (Streptococcus Pyogenes), Divides Regularly in Two Planes at RightMichael Vincent Pizarro BarbaNo ratings yet
- Presentation For Reporting in BiologyDocument61 pagesPresentation For Reporting in BiologyLovely Nhel EslomotNo ratings yet
- CELL Study MaterialDocument12 pagesCELL Study MaterialShyamasree SenguptaNo ratings yet
- Biology Fiitjee Chennai CentreDocument27 pagesBiology Fiitjee Chennai CentreT SunderamalolanNo ratings yet
- Chapter 4: Functional Anatomy of Prokaryotic and Eukaryotic CellsDocument94 pagesChapter 4: Functional Anatomy of Prokaryotic and Eukaryotic CellsTrevannie EdwardsNo ratings yet
- 2017-Bacterial Cell MechanicsDocument30 pages2017-Bacterial Cell MechanicsLAURA VALENTINA GUTIERREZ PINILLANo ratings yet
- Cell Structure and Funtions 2 PDFDocument49 pagesCell Structure and Funtions 2 PDFEllie MarrisNo ratings yet
- General Biology 1: Teacher: Reda QuidetDocument25 pagesGeneral Biology 1: Teacher: Reda QuidetJuanito MerciNo ratings yet
- BIO2107 - Tutorial 1 Discussion PointsDocument5 pagesBIO2107 - Tutorial 1 Discussion PointsAjay Sookraj RamgolamNo ratings yet
- 1.the Bacterial Cell Wall StructureDocument10 pages1.the Bacterial Cell Wall StructureMark Jhon MoradaNo ratings yet
- Bab 6 Sruktur Dan Fungsi Sel Bakteria (I)Document9 pagesBab 6 Sruktur Dan Fungsi Sel Bakteria (I)Shakira AmiraNo ratings yet
- Structure & Functions of Prokaryotic Cell. Bacteriophages.: Lecturer: - Sergei V. RedkozubovDocument54 pagesStructure & Functions of Prokaryotic Cell. Bacteriophages.: Lecturer: - Sergei V. RedkozubovJustineNo ratings yet
- Cellular Nanomechanics 2Document27 pagesCellular Nanomechanics 2Ahmed MotasimNo ratings yet
- Antibacterial and Anti Viral AgentsDocument51 pagesAntibacterial and Anti Viral AgentsabdeljelileNo ratings yet
- Prokaryotic and Eukaryotic Cells: Two Types of CellsDocument8 pagesProkaryotic and Eukaryotic Cells: Two Types of CellsMarcy BoralNo ratings yet
- 1214 Ijbes 01Document15 pages1214 Ijbes 01Breeze ChloeNo ratings yet
- Carbohydrate: The Cell WallDocument3 pagesCarbohydrate: The Cell WallFarah Fadhilah RosyadiNo ratings yet
- Microbiology Lec 1 PDFDocument7 pagesMicrobiology Lec 1 PDFAhmed AliNo ratings yet
- Mitosis and MeiosisDocument45 pagesMitosis and MeiosisJhon dave SurbanoNo ratings yet
- Microbiolgy 2nd LecDocument6 pagesMicrobiolgy 2nd Lecgailordfaker109No ratings yet
- Reviewer 4Document2 pagesReviewer 4AVILA JUDEEARLNo ratings yet
- Biology Unleashed: A Comprehensive Guide to Mastering the Science of LifeFrom EverandBiology Unleashed: A Comprehensive Guide to Mastering the Science of LifeNo ratings yet
- Ramachandran and His Map: C RamakrishnanDocument9 pagesRamachandran and His Map: C RamakrishnanchanduNo ratings yet
- Collagen - Natural Scaffold For Biology and EngineeringDocument11 pagesCollagen - Natural Scaffold For Biology and EngineeringStefany CondorNo ratings yet
- Biochemistry Notes ProteinsDocument6 pagesBiochemistry Notes ProteinsRegine Chua100% (1)
- Surface Sciences: Springer Series inDocument415 pagesSurface Sciences: Springer Series inFaidra AmargianouNo ratings yet
- Csir Chemistry Previous Years Questions With Answer PDFDocument187 pagesCsir Chemistry Previous Years Questions With Answer PDFBin RenNo ratings yet
- CharlesCantorandPaulSchimmel BiophysicalChemistry PartI TheConformationofBiologicalMacromoleculesBiophysicalChemistry1980W.H.freeman Libgen - Li1Document408 pagesCharlesCantorandPaulSchimmel BiophysicalChemistry PartI TheConformationofBiologicalMacromoleculesBiophysicalChemistry1980W.H.freeman Libgen - Li1Riya gupta100% (1)
- Biol 130 Notes 2012Document121 pagesBiol 130 Notes 2012Nick O'HaraNo ratings yet
- Collagen StructureDocument42 pagesCollagen StructureAatmaanandaNo ratings yet
- Proteins 1Document131 pagesProteins 1EyaNo ratings yet
- L 17 Structure and Functions of ProteinsDocument43 pagesL 17 Structure and Functions of ProteinssNo ratings yet
- 11 - Biochemistry MCQs Amino Acid & PROTEINSDocument8 pages11 - Biochemistry MCQs Amino Acid & PROTEINSMohamed YahiaNo ratings yet
- Primary Structure of A ProteinDocument2 pagesPrimary Structure of A ProteinNkanyiso Mondli ZunguNo ratings yet
- Protein StructureDocument42 pagesProtein StructureronojoysenguptaNo ratings yet
- 5) Qualitative Tests of ProteinsDocument8 pages5) Qualitative Tests of ProteinsSPMUSER9ANo ratings yet
- Strands and SheetsDocument19 pagesStrands and SheetsDaniel TanNo ratings yet
- MCQ On Molecular BiologyDocument12 pagesMCQ On Molecular Biologyronojoysengupta0% (1)
- FST3107-INTRODUCTION TO FOOD CHEMISTRY - ProteinDocument67 pagesFST3107-INTRODUCTION TO FOOD CHEMISTRY - ProteinZHOU TIANLENo ratings yet
- Peptide BondDocument2 pagesPeptide BondA PutoyNo ratings yet
- 2 QDocument110 pages2 QEnrique PugaNo ratings yet
- Hood-DeGrenier 2015 Active Learning Workshops - Protein Structure WorkshopDocument12 pagesHood-DeGrenier 2015 Active Learning Workshops - Protein Structure WorkshopPriya KumarNo ratings yet
- Lipid Movement BiochemDocument9 pagesLipid Movement BiochemCrowNo ratings yet
- 2022 - HULGAN and HARTEINK - Recent Advances in Collagen Mimetic Peptide Structure and DesignDocument15 pages2022 - HULGAN and HARTEINK - Recent Advances in Collagen Mimetic Peptide Structure and DesignCarlos FerreiraNo ratings yet
- (BIF 401) Current Solved Papers.Document16 pages(BIF 401) Current Solved Papers.Sagheer MalikNo ratings yet
- BIOCHEM 3 Three Dimensional Structure of ProteinsDocument4 pagesBIOCHEM 3 Three Dimensional Structure of ProteinsRudolph MendozaNo ratings yet
- Biochemistry Proteins MidtermDocument9 pagesBiochemistry Proteins MidtermCarla Marie LedaNo ratings yet