Professional Documents
Culture Documents
Science SPM: Glucose Yeast X + Carbon Dioxide
Science SPM: Glucose Yeast X + Carbon Dioxide
Uploaded by
InerTiaOpheliaCopyright:
Available Formats
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5823)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- 1963 Ford GalaxieDocument190 pages1963 Ford GalaxieLuis100% (2)
- Task 1 - Model AnswerDocument4 pagesTask 1 - Model AnswerShauryaNo ratings yet
- Newton Raphson MethodDocument29 pagesNewton Raphson Methodfaizankhan23100% (1)
- BUSINESS COMMUNICATION Assignment AIMADocument8 pagesBUSINESS COMMUNICATION Assignment AIMAAkash Choudhury67% (6)
- Emerging Space MarketsDocument153 pagesEmerging Space MarketsAlexandru Toncu100% (1)
- Expoeriment f4Document11 pagesExpoeriment f4Suriati Bt A RashidNo ratings yet
- Program Jejak Jaya Kimia Modul Kertas 3 Soalan 3Document2 pagesProgram Jejak Jaya Kimia Modul Kertas 3 Soalan 3Suriati Bt A RashidNo ratings yet
- Chapter 6 - Nuclear Energy: What Is RadioactiveDocument2 pagesChapter 6 - Nuclear Energy: What Is RadioactiveSuriati Bt A RashidNo ratings yet
- Mind Mapping TemplateDocument15 pagesMind Mapping TemplateSuriati Bt A RashidNo ratings yet
- Trial Paper Chemistry p3 DDocument6 pagesTrial Paper Chemistry p3 DSuriati Bt A RashidNo ratings yet
- Yearly Plan Chemistry Form 4 2014: SMK Katholik, 28700 Bentong Pahang Darul MakmurDocument31 pagesYearly Plan Chemistry Form 4 2014: SMK Katholik, 28700 Bentong Pahang Darul MakmurSuriati Bt A RashidNo ratings yet
- 2010 P3 Teacher SheetDocument3 pages2010 P3 Teacher SheetSuriati Bt A RashidNo ratings yet
- Bengkel Dan Teknik Menjawab SPM 2011: 1511/2 Science Paper 2 2 HoursDocument15 pagesBengkel Dan Teknik Menjawab SPM 2011: 1511/2 Science Paper 2 2 HoursHairolhamzi Bin BahroddinNo ratings yet
- Contoh Sijil Peka SainsDocument2 pagesContoh Sijil Peka SainsSuriati Bt A RashidNo ratings yet
- Borang Gred Induk PEKA Sains SPMDocument3 pagesBorang Gred Induk PEKA Sains SPMRadzuan Mokhtar Ruddin100% (1)
- Terangkan Jawapan Anda Berdasarkan Perkara Berikut : Choose The Vehicle Which Has More Safety FeaturesDocument4 pagesTerangkan Jawapan Anda Berdasarkan Perkara Berikut : Choose The Vehicle Which Has More Safety FeaturesSuriati Bt A RashidNo ratings yet
- Modul Section C Q10Document6 pagesModul Section C Q10Suriati Bt A RashidNo ratings yet
- Pencil Final f4Document16 pagesPencil Final f4Suriati Bt A RashidNo ratings yet
- T3 Bab 1Document10 pagesT3 Bab 1Khairiyah AbdullahNo ratings yet
- Instant PotDocument48 pagesInstant PotCatrinescu Oana100% (1)
- Haryana at A Glance: Geographical AreaDocument1 pageHaryana at A Glance: Geographical AreasonuNo ratings yet
- NOTH Menu Aug 22Document10 pagesNOTH Menu Aug 22Devin SwanepoelNo ratings yet
- Product Strategy of Maruti SuzukiDocument42 pagesProduct Strategy of Maruti SuzukiIndira ThayilNo ratings yet
- Kings Gambit PrimerDocument5 pagesKings Gambit PrimerdlNo ratings yet
- MCQ Test of PolymerDocument4 pagesMCQ Test of PolymerDrAman Khan Pathan100% (2)
- Iygb Gce: Core Mathematics C2 Advanced SubsidiaryDocument6 pagesIygb Gce: Core Mathematics C2 Advanced SubsidiaryssmithNo ratings yet
- Urban HierarchyDocument12 pagesUrban HierarchyNafisa Irina100% (1)
- Atkins Diet MenuDocument6 pagesAtkins Diet MenuSohaila KhaledNo ratings yet
- THE LAW PERTAINING TO PRIVATE PERSONAL & COMML RELATIONS - AdDU Bar NotesDocument54 pagesTHE LAW PERTAINING TO PRIVATE PERSONAL & COMML RELATIONS - AdDU Bar NotesDevilleres Eliza DenNo ratings yet
- Profitability and Marketability of The Top 55 U.S. Commercial BanksDocument19 pagesProfitability and Marketability of The Top 55 U.S. Commercial BanksSasa LuNo ratings yet
- Start-Up Report Warranty Registration - EDITABLEDocument1 pageStart-Up Report Warranty Registration - EDITABLESAED ALAWNEHNo ratings yet
- Linguistics of Ancient India and Its Main DirectionsDocument8 pagesLinguistics of Ancient India and Its Main DirectionsastaNo ratings yet
- NSRP Refinery Plant Environmental Impact AssessmentDocument115 pagesNSRP Refinery Plant Environmental Impact AssessmentScribd_del88% (8)
- Engine RT-flex Operation Practical AdvancedDocument3 pagesEngine RT-flex Operation Practical AdvancedPiotr StankiewiczNo ratings yet
- Study of Adhesion Properties of Natural Rubber, Epoxidized Natural Rubber, and Ethylene-Propylene Diene Terpolymer-Based AdhesivesDocument44 pagesStudy of Adhesion Properties of Natural Rubber, Epoxidized Natural Rubber, and Ethylene-Propylene Diene Terpolymer-Based AdhesivesZarathos SinghNo ratings yet
- Certificate: Internal External PrincipalDocument36 pagesCertificate: Internal External PrincipalNitesh kuraheNo ratings yet
- Tle Ia Q4 Week 5Document46 pagesTle Ia Q4 Week 5Joevelyn Adaron GalayNo ratings yet
- New Hptu Syllabus CSEDocument138 pagesNew Hptu Syllabus CSEerankursharma1985No ratings yet
- Draft Spec. For Set of Panels For LHB Non AC EOG Coaches PDFDocument27 pagesDraft Spec. For Set of Panels For LHB Non AC EOG Coaches PDFVishal Kinikar0% (1)
- CAR-T TherapyDocument14 pagesCAR-T TherapyrameshaachariarNo ratings yet
- GE-102 - Natural Sciences - OutlinesDocument3 pagesGE-102 - Natural Sciences - Outlinesm.zulaid.motivationNo ratings yet
- The 10 Commandments (MonkeyDM)Document45 pagesThe 10 Commandments (MonkeyDM)Claudio GonzalezNo ratings yet
- Introduction SlidesDocument23 pagesIntroduction SlidesMathiselvan GopalNo ratings yet
- Supplimentary Material: LU DecompositionDocument14 pagesSupplimentary Material: LU DecompositionGilNo ratings yet
Science SPM: Glucose Yeast X + Carbon Dioxide
Science SPM: Glucose Yeast X + Carbon Dioxide
Uploaded by
InerTiaOpheliaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Science SPM: Glucose Yeast X + Carbon Dioxide
Science SPM: Glucose Yeast X + Carbon Dioxide
Uploaded by
InerTiaOpheliaCopyright:
Available Formats
HIKMAH MODULE
SCIENCE SPM
MODULE 11
PAPER 1
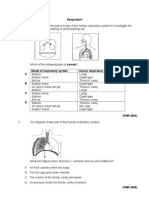
1. Diagram 1 shows an experiment to produce ethanol.
Diagram 1
What is the process involved?
A.
B.
C.
D.
Oxidation
Distillation
Fermentation
Vulcanization
2. Diagram 2 shows the equation represents a reaction.
Glucose
yeast
X +
Diagram 2
Which of the match is correct?
Process
A.
B.
C.
D.
Esterification
Fermentation
Respiration
Photosynthesis
X
Ester
Alcohol
Water
Oxygen
110
Carbon dioxide
HIKMAH MODULE
3. Diagram 3 shows an experiment to produce soap in a laboratory.
Diagram 3
What is solution Q?
A. Water
B. Ethyl ethanoate
C. Hydrochloric acid
Sodium hydroxide
4.
Diagram 4 shows a process.
Diagram 4
What is process X?
A.
B.
C.
D.
Oxidation
Polymerisation
Neutralisation
Depolymerisation
111
HIKMAH MODULE
5. Diagram 5 shows the structure of a polymer before and after process X.
Diagram 5
What is process X?
A.
B.
C.
D.
Oxidation
Coagulation
Vulcanisation
Neutralisation
6. Which of the following is a natural polymer?
A.
B.
C.
D.
Nylon
Protein
Glucose
Amino acid
7. Diagram 6 shows a molecule of a substance.
Diagram 6
What is the substance?
A.
B.
C.
D.
Fat
Soap
Latex
Ester
112
HIKMAH MODULE
8. Why does ammonia solution can prevent latex from coagulating?
A.
B.
C.
D.
Ammonia separates rubber molecule from one another.
Ammonia harden the membrane that covering rubber molecules.
Positive ions in ammonia solution neutralize negative ions in latex.
Negative ions in ammonia solution neutralize positive ions in latex
that produced by the action of bacteria.
9. Diagram 7 shows the changes in structure of a rubber sheet when stretched and
then released.
Diagram 7
Which of the characteristics explain the situation?
A.
B.
C.
D.
Soft
Hard
Sticky
Elastic
113
HIKMAH MODULE
10. Diagram 8 shows the structure of a plastic.
Diagram 8
Which of the following is probably the type of plastic?
A.
B.
C.
D.
Epoxy
Bakelit
Perspex
Melamine
114
HIKMAH MODULE
PAPER 2
SECTION A + B
1
Diagram 1 shows an experiment to study the amount of carbon dioxide released
in the fermentation process.
DIAGRAM 1
Table 1 shows the result of the experiment
Time taken/ day
Volume of gas /cm3
(a)
(i)
0
0
1
10
Table 1
2
20
3
25
5
30
Using data in table 1 above, draw the graph of volume of gas
against time taken.
Volume of gas /cm3
30
25
20
15
10
5
115
Time taken /Day
HIKMAH MODULE
(ii)
Base on the graph, state the volume of carbon dioxide released
on the fourth day?
..
(b)
What is the relationship between the volume of carbon dioxide gas?
released and time taken?
.
(b)
Predict the volume of carbon dioxide gas released on the sixth day?
2. Diagram 1.1 and 1.2 show an experiment to produce ethanol through fermentation
process.
Diagram 1.1
Diagram 1.2
a) Based on Diagram 1.2, what is your observation on the lime water after a few
hours?
116
HIKMAH MODULE
b) State hypothesis that can be made from this experiment.
c) State the variables in this experiment.
(i)
Fixed
variable:
(ii)
Manipulated variable:..
d) Mark ( / ) the substances that contain alcohol.
Milk
Wine
Perfume
3. Figure shows a section through the oil palm fruit.
P
Q
R
a) Name the parts labeled P and Q.
P:
Q:
117
HIKMAH MODULE
b) Which part produces more palm oil?
..
c) What type of oil is contained in the oil palm?
..
d) State one advantage of using palm oil.
.
4.
Type of rubber
Natural rubber
Vulcanised rubber
Initial length
5.0
5.0
Length (cm)
Length with weight
Final length without weight
8.0
6.0
7.0
5.0
a. State a hypothesis for this experiment.
b. State the:
Manipulated variable:
Responding variable :..
Diagram 1 shows how latex is turned into vulcanized rubber.
Latex
+P
Coagulated latex
+Q
Vulcanised rubber
Diagram 1
a) Name the substance:
(i) P:
(ii) Q:
b) State one difference between natural rubber and vulcanized rubber.
....
118
HIKMAH MODULE
c) Draw the molecular structure of the vulcanized rubber.
Heated with Q
Natural rubber
Vulcanised rubber
SECTION C
1.a) Malaysia exports raw rubber sheets and rubber latex Rubber latex is tapped and
collected from plantation throughout the country.
Suggest and explain:
i)
how rubber sheets are produced
ii)
rubber latex is prevented from coagulating
[4 marks]
b) The main use of natural rubber is to manufacture car tyres. However, it is found that
natural rubber is soft and not resistant to heat which is sticky when heated. Hence, it is
not suitable for making car tyres.
Explain how the quality of natural rubber can be improved so that it can be used to
make car tyres. Your answer should include the following:
Identifying the problem
Clarification of the problem
Suggest method to solve the problem
Explain how the method can solve the problem
[6 marks]
119
HIKMAH MODULE
MODULE 11 - ANSWERS
PAPER 1
1. C
2. B
3. D
4. D
5. C
6. B
7. C
8. D
9. D
10. C
PAPER 2
SECTION A + B
1.(a)(i)
(a)
(b)
(b)
(ii)
28 + 0.5 (cm3)
The longer the time taken, the higher the volume of carbon dioxide gas
released
32(cm3)
2.
a) The lime water turns cloudy
b) Ethanol is produced by the action of sugar solution and yeast// Ethanol is
produced through fermentation process// carbon dioxide is produced from
fermentation process
c) (i) Fixed variable: Volume of sugar solution// Quantity of sugar solution
(ii)Manipulated variable : Presence of yeast// Type of solution
d) Able to tick on Wine and perfume
120
HIKMAH MODULE
3. a) P: mesocarp
Q: kernel
b) mesocarp // P
c) Unsaturated fat
d) Contain vitamin A // E // no cholesterol
4. a) (i) ethanoic acid
(ii) sulphur
b) Vulcanized rubber is : more elastic than natural rubber//
harder//
stronger//
more resistant to heat
c)
SECTION C
1.a) (i) Add ethanoic acid into the latex
- Hydrogen ions in acid neutralize negative charge on protein membrane, causes
the rubber particles to collide, protein membrane break and rubber molecules released
and combined.
(ii) Add ammonia solution into the latex
- Negatively charges hydroxyl ion in ammonia neutralize the hydrogen ion in latex
produced by action of bacteria.
b) * Problem:
- not suitable for making tyres
* Clarification of the problem:
- Natural rubber is soft and not resistant to heat.
* Method to solve the problem:
- through the process of vulcanization
* Explanation:
- natural rubber is heated with sulphur.
- The sulphur atoms will form cross-linkage between the rubber molecules.
- This make vulcanized rubber harder and more resistant to heat.
121
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5823)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- 1963 Ford GalaxieDocument190 pages1963 Ford GalaxieLuis100% (2)
- Task 1 - Model AnswerDocument4 pagesTask 1 - Model AnswerShauryaNo ratings yet
- Newton Raphson MethodDocument29 pagesNewton Raphson Methodfaizankhan23100% (1)
- BUSINESS COMMUNICATION Assignment AIMADocument8 pagesBUSINESS COMMUNICATION Assignment AIMAAkash Choudhury67% (6)
- Emerging Space MarketsDocument153 pagesEmerging Space MarketsAlexandru Toncu100% (1)
- Expoeriment f4Document11 pagesExpoeriment f4Suriati Bt A RashidNo ratings yet
- Program Jejak Jaya Kimia Modul Kertas 3 Soalan 3Document2 pagesProgram Jejak Jaya Kimia Modul Kertas 3 Soalan 3Suriati Bt A RashidNo ratings yet
- Chapter 6 - Nuclear Energy: What Is RadioactiveDocument2 pagesChapter 6 - Nuclear Energy: What Is RadioactiveSuriati Bt A RashidNo ratings yet
- Mind Mapping TemplateDocument15 pagesMind Mapping TemplateSuriati Bt A RashidNo ratings yet
- Trial Paper Chemistry p3 DDocument6 pagesTrial Paper Chemistry p3 DSuriati Bt A RashidNo ratings yet
- Yearly Plan Chemistry Form 4 2014: SMK Katholik, 28700 Bentong Pahang Darul MakmurDocument31 pagesYearly Plan Chemistry Form 4 2014: SMK Katholik, 28700 Bentong Pahang Darul MakmurSuriati Bt A RashidNo ratings yet
- 2010 P3 Teacher SheetDocument3 pages2010 P3 Teacher SheetSuriati Bt A RashidNo ratings yet
- Bengkel Dan Teknik Menjawab SPM 2011: 1511/2 Science Paper 2 2 HoursDocument15 pagesBengkel Dan Teknik Menjawab SPM 2011: 1511/2 Science Paper 2 2 HoursHairolhamzi Bin BahroddinNo ratings yet
- Contoh Sijil Peka SainsDocument2 pagesContoh Sijil Peka SainsSuriati Bt A RashidNo ratings yet
- Borang Gred Induk PEKA Sains SPMDocument3 pagesBorang Gred Induk PEKA Sains SPMRadzuan Mokhtar Ruddin100% (1)
- Terangkan Jawapan Anda Berdasarkan Perkara Berikut : Choose The Vehicle Which Has More Safety FeaturesDocument4 pagesTerangkan Jawapan Anda Berdasarkan Perkara Berikut : Choose The Vehicle Which Has More Safety FeaturesSuriati Bt A RashidNo ratings yet
- Modul Section C Q10Document6 pagesModul Section C Q10Suriati Bt A RashidNo ratings yet
- Pencil Final f4Document16 pagesPencil Final f4Suriati Bt A RashidNo ratings yet
- T3 Bab 1Document10 pagesT3 Bab 1Khairiyah AbdullahNo ratings yet
- Instant PotDocument48 pagesInstant PotCatrinescu Oana100% (1)
- Haryana at A Glance: Geographical AreaDocument1 pageHaryana at A Glance: Geographical AreasonuNo ratings yet
- NOTH Menu Aug 22Document10 pagesNOTH Menu Aug 22Devin SwanepoelNo ratings yet
- Product Strategy of Maruti SuzukiDocument42 pagesProduct Strategy of Maruti SuzukiIndira ThayilNo ratings yet
- Kings Gambit PrimerDocument5 pagesKings Gambit PrimerdlNo ratings yet
- MCQ Test of PolymerDocument4 pagesMCQ Test of PolymerDrAman Khan Pathan100% (2)
- Iygb Gce: Core Mathematics C2 Advanced SubsidiaryDocument6 pagesIygb Gce: Core Mathematics C2 Advanced SubsidiaryssmithNo ratings yet
- Urban HierarchyDocument12 pagesUrban HierarchyNafisa Irina100% (1)
- Atkins Diet MenuDocument6 pagesAtkins Diet MenuSohaila KhaledNo ratings yet
- THE LAW PERTAINING TO PRIVATE PERSONAL & COMML RELATIONS - AdDU Bar NotesDocument54 pagesTHE LAW PERTAINING TO PRIVATE PERSONAL & COMML RELATIONS - AdDU Bar NotesDevilleres Eliza DenNo ratings yet
- Profitability and Marketability of The Top 55 U.S. Commercial BanksDocument19 pagesProfitability and Marketability of The Top 55 U.S. Commercial BanksSasa LuNo ratings yet
- Start-Up Report Warranty Registration - EDITABLEDocument1 pageStart-Up Report Warranty Registration - EDITABLESAED ALAWNEHNo ratings yet
- Linguistics of Ancient India and Its Main DirectionsDocument8 pagesLinguistics of Ancient India and Its Main DirectionsastaNo ratings yet
- NSRP Refinery Plant Environmental Impact AssessmentDocument115 pagesNSRP Refinery Plant Environmental Impact AssessmentScribd_del88% (8)
- Engine RT-flex Operation Practical AdvancedDocument3 pagesEngine RT-flex Operation Practical AdvancedPiotr StankiewiczNo ratings yet
- Study of Adhesion Properties of Natural Rubber, Epoxidized Natural Rubber, and Ethylene-Propylene Diene Terpolymer-Based AdhesivesDocument44 pagesStudy of Adhesion Properties of Natural Rubber, Epoxidized Natural Rubber, and Ethylene-Propylene Diene Terpolymer-Based AdhesivesZarathos SinghNo ratings yet
- Certificate: Internal External PrincipalDocument36 pagesCertificate: Internal External PrincipalNitesh kuraheNo ratings yet
- Tle Ia Q4 Week 5Document46 pagesTle Ia Q4 Week 5Joevelyn Adaron GalayNo ratings yet
- New Hptu Syllabus CSEDocument138 pagesNew Hptu Syllabus CSEerankursharma1985No ratings yet
- Draft Spec. For Set of Panels For LHB Non AC EOG Coaches PDFDocument27 pagesDraft Spec. For Set of Panels For LHB Non AC EOG Coaches PDFVishal Kinikar0% (1)
- CAR-T TherapyDocument14 pagesCAR-T TherapyrameshaachariarNo ratings yet
- GE-102 - Natural Sciences - OutlinesDocument3 pagesGE-102 - Natural Sciences - Outlinesm.zulaid.motivationNo ratings yet
- The 10 Commandments (MonkeyDM)Document45 pagesThe 10 Commandments (MonkeyDM)Claudio GonzalezNo ratings yet
- Introduction SlidesDocument23 pagesIntroduction SlidesMathiselvan GopalNo ratings yet
- Supplimentary Material: LU DecompositionDocument14 pagesSupplimentary Material: LU DecompositionGilNo ratings yet