Professional Documents
Culture Documents
SRP Table Chem Data
SRP Table Chem Data
Uploaded by
api-222503660Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SRP Table Chem Data
SRP Table Chem Data
Uploaded by
api-222503660Copyright:
Available Formats
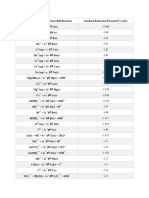
Standard Reduction Potentials at 25 C
Half-reaction
F2(g) + 2 e
H2O2(aq) + 2 H+(aq) + 2 e
PbO2(s) + SO42(aq) + 4 H+(aq) + 2 e
E(volts)
2 F (aq)
+ 2.89
2 H2
+ 1.76
PbSO4(s) + 2 H2
+ 1.69
(aq) + 2 e
(g) + 2 H2
+ 1.63
MnO (aq) + 8 H (aq) + 5 e
Mn (aq) + 4 H2
+ 1.51
Au (aq) + 3 e
Au(s)
+ 1.50
(aq) + 2 e
(aq) + H2
+ 1.49
PbO2(s) + 4 H (aq) + 2 e
Pb (aq) + 2 H2
+ 1.46
(aq)
+ 1.36
3+
+
+
2+
2+
(g) + 2 e
2
Cr2O (aq) + 14 H (aq) + 6 e
2 Cr (aq) + 7 H2
+ 1.36
O2(g) + 4 H (aq) + 4 e
2 H2
+ 1.23
2
7
+
+
3+
2 Br (aq)
+ 1.08
Ag (aq) + e
Ag(s)
+ 0.80
Fe (aq) + e
Br2
+
Fe (aq)
+ 0.77
H2O2(aq)
+ 0.70
2 I(aq)
+ 0.54
4 OH (aq)
+ 0.40
Cu (aq) + 2 e
Cu(s)
+ 0.34
S(s)+ 2 H (aq) + 2 e
H2S(aq)
+ 0.17
2 H (aq) + 2 e
H2(g)
0 exactly
Pb2+(aq) + 2 e
Pb(s)
0.13
Sn2+(aq) + 2 e
Sn(s)
0.14
Ni (aq) + 2 e
Ni(s)
0.24
Co (aq) + 2 e
Co(s)
3+
O2(g) + 2 H+(aq) + 2 e
I2(s) + 2 e
O2(g) + 2 H2
2+
+
+
2+
2+
2+
0.28
Pb(s) + SO (aq)
0.36
Cd (aq) + 2 e
Cd(s)
0.40
2 CO2(g) + 2 H (aq) + 2 e
PbSO4(s) + 2 e
2+
2
4
H2C2O4(aq)
0.43
Fe2+(aq) + 2 e
Fe(s)
0.44
Cr (aq) + 3 e
Cr(s)
0.74
2+
Zn (aq) + 2 e
Zn(s)
2 H2
H2(g) + 2 OH (aq)
0.83
Mn (aq) + 2 e
Mn(s)
1.18
(aq) + 3 e
3+
2+
3+
0.76
1.68
Mg(s)
2.36
Na (aq) + e
Na(s)
2.71
Ca (aq) + 2 e
Ca(s)
2.87
Sr (aq) + 2 e
Sr(s)
2.90
Ba (aq) + 2 e
Ba(s)
2.91
K (aq) + e
K(s)
2.94
Mg2+(aq) + 2 e
+
2+
2+
2+
[Data source: Aylward, G.H., & Findlay, T. (2008). SI Chemical Data (6th ed.). Queensland: John Wiley & Sons Australia, Ltd.]
You might also like
- Standard Reduction PotentialsDocument3 pagesStandard Reduction PotentialsjaverfrivNo ratings yet
- Standard Reduction Potentials Data Extended PDFDocument2 pagesStandard Reduction Potentials Data Extended PDFAceNo ratings yet
- Chemistry Stage 2 and 3 Data Sheet 2010Document4 pagesChemistry Stage 2 and 3 Data Sheet 2010Edy LiewNo ratings yet
- Standard Electrode Potential SeriesDocument1 pageStandard Electrode Potential SeriesWONG KEE PING MoeNo ratings yet
- Standard Reduction PotentialDocument7 pagesStandard Reduction Potentialyoyotoonzone1No ratings yet
- ElectrodeDocument2 pagesElectrodeThatcher PanchoNo ratings yet
- Chapter 3 Oxidation ReductionDocument68 pagesChapter 3 Oxidation Reductionlong.vuongbz188No ratings yet
- Reduction Half-Reaction E (V) : Neutral or Acid SolutionDocument3 pagesReduction Half-Reaction E (V) : Neutral or Acid SolutionEric FernandoNo ratings yet
- DebateDocument3 pagesDebatebbangeles1No ratings yet
- Appendix EDocument1 pageAppendix EYrhon IbanezNo ratings yet
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFalbi veshiNo ratings yet
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFAlexander Salado IbrahimNo ratings yet
- Red Ox AnswersDocument2 pagesRed Ox Answerspaulmutambo509No ratings yet
- Extract 10 PagesDocument10 pagesExtract 10 PageskuoklukeNo ratings yet
- Chapter 4 Oxidation-ReductionDocument68 pagesChapter 4 Oxidation-ReductionPHƯƠNG ĐẶNG YẾNNo ratings yet
- SOA and SRA TableDocument1 pageSOA and SRA TableAhhhhhhhhhhhNo ratings yet
- HalfCellPoten TableDocument1 pageHalfCellPoten TableJeisson David Sanchez PInzonNo ratings yet
- Standard Electrode Potentials in Aqueous Solution at 25°C: TablesDocument2 pagesStandard Electrode Potentials in Aqueous Solution at 25°C: TablesLouie G NavaltaNo ratings yet
- Standardreductionpotentials PDFDocument1 pageStandardreductionpotentials PDFEsat GoceriNo ratings yet
- Standard Reduction PotentialsDocument1 pageStandard Reduction PotentialsAnonymous s4HW3TX0IHNo ratings yet
- Standardreductionpotential PDFDocument1 pageStandardreductionpotential PDFShiizan123No ratings yet
- Standard Reduction PotentialDocument1 pageStandard Reduction PotentialghanifdkdNo ratings yet
- Standard Reduction Potential TablesDocument1 pageStandard Reduction Potential TablesJenver NanquiladaNo ratings yet
- 233 SolutionsDocument11 pages233 Solutionsestellasr00No ratings yet
- Standard Reduction (Electrode) PotentialsDocument2 pagesStandard Reduction (Electrode) Potentialsbackch9011No ratings yet
- CHEM1 Datasheet May 2020Document4 pagesCHEM1 Datasheet May 2020Miku HatsuneNo ratings yet
- Topic 13 Exercise 3 - Spontaneous ReactionsDocument1 pageTopic 13 Exercise 3 - Spontaneous ReactionsdenisNo ratings yet
- Standard Reduction PotentialsDocument5 pagesStandard Reduction PotentialsnathaloaNo ratings yet
- Silo - Tips 2 Write The Chemical Formulas of The Products and Balance The Following Spontaneous ReactionsDocument40 pagesSilo - Tips 2 Write The Chemical Formulas of The Products and Balance The Following Spontaneous ReactionsAkash BhoiNo ratings yet
- Standard Electrode Potentials in Aqueous Solution at 25Document2 pagesStandard Electrode Potentials in Aqueous Solution at 25Ani Devi AriyantiNo ratings yet
- Problems For Balancing of Redox ReactionsDocument1 pageProblems For Balancing of Redox ReactionsUtsavNo ratings yet
- The Dien Cuc ChuanDocument9 pagesThe Dien Cuc Chuanvinasat1108No ratings yet
- Chem 2Document8 pagesChem 22021302095No ratings yet
- Standard Electrode PotentialDocument11 pagesStandard Electrode PotentialRSLNo ratings yet
- Writing Chemical Equations: Test Yourself 8.1 (Page 130)Document1 pageWriting Chemical Equations: Test Yourself 8.1 (Page 130)Zeeshan MunirNo ratings yet
- Sap 5Document22 pagesSap 5reza noviyantiNo ratings yet
- P2 Standard Reduction Potentials by ValueDocument6 pagesP2 Standard Reduction Potentials by ValueASTRID ELIZABET CUEVA GUTIERREZNo ratings yet
- ReaksiDocument2 pagesReaksiherna watiNo ratings yet
- Chemical Equations: Preparation For College Chemistry Columbia University Department of ChemistryDocument31 pagesChemical Equations: Preparation For College Chemistry Columbia University Department of ChemistryLakshmi SinghNo ratings yet
- Chem16 Experiment # 1: Chemical Changes Post-Lab Discussion: 3 2 (Aq) (Aq) 2 (S) 3 (Aq) 2+ (Aq) - (Aq) 2 (S)Document1 pageChem16 Experiment # 1: Chemical Changes Post-Lab Discussion: 3 2 (Aq) (Aq) 2 (S) 3 (Aq) 2+ (Aq) - (Aq) 2 (S)DiyanikaNo ratings yet
- IoneqwrkshtDocument2 pagesIoneqwrkshtpixniteplayzNo ratings yet
- ReaccionesDocument3 pagesReaccionesJarek Jhoel Alejandro ZarateNo ratings yet
- STD Electrode PontentialsDocument3 pagesSTD Electrode PontentialsVishal PamnaniNo ratings yet
- ch12 Odd PDFDocument37 pagesch12 Odd PDFmecsolNo ratings yet
- IONIC EQUATIONS AnswersDocument1 pageIONIC EQUATIONS AnswersAlex noslenNo ratings yet
- Chem 110 Quiz BalancingDocument1 pageChem 110 Quiz BalancinglucasNo ratings yet
- Edexecel IAL Lesson 1Document20 pagesEdexecel IAL Lesson 1Pevin De silvaNo ratings yet
- Half and Ionic Equations (GCSE)Document31 pagesHalf and Ionic Equations (GCSE)william.ongeri.tutoringNo ratings yet
- Standardreductionpotentials PDFDocument1 pageStandardreductionpotentials PDFBadrus SyamsiNo ratings yet
- Bab 6Document31 pagesBab 6Timothy HillNo ratings yet
- Balancing Redox Reactions WorksheetDocument2 pagesBalancing Redox Reactions Worksheetsai sreerama mNo ratings yet
- SUSA, ALVIN II B - 1BSIE-B - WEEK-3-ActivityDocument5 pagesSUSA, ALVIN II B - 1BSIE-B - WEEK-3-ActivityAlbert MariquitNo ratings yet
- Reactions For Preparation of A Few GasesDocument1 pageReactions For Preparation of A Few Gasesraamki_99No ratings yet
- Chapter 5 Answers Practice Examples: ReductionDocument7 pagesChapter 5 Answers Practice Examples: ReductionEmre Enes EdizNo ratings yet
- CH 02Document17 pagesCH 02Simay OğuzkurtNo ratings yet
- Ch12 Redox Ws Keys 1 13Document28 pagesCh12 Redox Ws Keys 1 13Allen IBARRA VILLAMINNo ratings yet
- Standard Electrode PotentialDocument14 pagesStandard Electrode PotentialFahrur RoziNo ratings yet
- Unit 2 - The Essential EquationsDocument2 pagesUnit 2 - The Essential EquationsFrihah AkhtarNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Nitrogencyclebuilds ProteinsDocument3 pagesNitrogencyclebuilds Proteinsapi-222503660No ratings yet
- Investigation Design TemplateDocument4 pagesInvestigation Design Templateapi-222503660No ratings yet
- 2018 01 Organic Chemistry Introductory AnalysisDocument1 page2018 01 Organic Chemistry Introductory Analysisapi-222503660No ratings yet
- Carbon Cycle Facilitates Energy FlowDocument2 pagesCarbon Cycle Facilitates Energy Flowapi-222503660No ratings yet
- 2018 Assess Task 2 Writing Ecosystem TaskbriefDocument2 pages2018 Assess Task 2 Writing Ecosystem Taskbriefapi-222503660No ratings yet
- 2018 Integratedscience Program Semester 1 Y11 2017Document10 pages2018 Integratedscience Program Semester 1 Y11 2017api-222503660No ratings yet
- 2018 Task 3Document1 page2018 Task 3api-222503660No ratings yet
- Dear Wa Science TeachersDocument1 pageDear Wa Science Teachersapi-222503660No ratings yet
- 2018 Assess Task 2 Ecosystem FieldnotesDocument4 pages2018 Assess Task 2 Ecosystem Fieldnotesapi-222503660No ratings yet
- 2017 Integratedscience Physics Program Y11 2017 Sem2Document1 page2017 Integratedscience Physics Program Y11 2017 Sem2api-222503660No ratings yet
- 2018 01 Labskills Solution TransferDocument2 pages2018 01 Labskills Solution Transferapi-222503660No ratings yet
- Task 16 Planetarty Pasta Rover Build and TestDocument2 pagesTask 16 Planetarty Pasta Rover Build and Testapi-222503660No ratings yet
- 2017 Assessmenttask2 Design Portable Worm Farm TaskbriefcompleteDocument6 pages2017 Assessmenttask2 Design Portable Worm Farm Taskbriefcompleteapi-222503660No ratings yet
- Task 15 Planetary RoversDocument2 pagesTask 15 Planetary Roversapi-222503660No ratings yet
- 2017 Assessmenttask12 Designchemicalreactiondemonstration Taskbrief NoimagesDocument2 pages2017 Assessmenttask12 Designchemicalreactiondemonstration Taskbrief Noimagesapi-222503660No ratings yet
- Integrated-Science-Y11-Syllabus-General-2016editedfor Litaeracy FocusDocument3 pagesIntegrated-Science-Y11-Syllabus-General-2016editedfor Litaeracy Focusapi-222503660No ratings yet
- Tripling or Tritosing The TablesDocument3 pagesTripling or Tritosing The Tablesapi-222503660No ratings yet
- 2017 Assessmenttask3and4 Designcellorganelle TaskbriefDocument5 pages2017 Assessmenttask3and4 Designcellorganelle Taskbriefapi-222503660No ratings yet
- 2017 Assessmenttask3and4 Designcellorganelle TaskbriefDocument5 pages2017 Assessmenttask3and4 Designcellorganelle Taskbriefapi-222503660No ratings yet
- 2017 Stem Assessmenttask Design A Portable Worm Farm Taskbrief RelevantsyllabusstatementsDocument1 page2017 Stem Assessmenttask Design A Portable Worm Farm Taskbrief Relevantsyllabusstatementsapi-222503660No ratings yet
- 01 CellstructurefunctionDocument18 pages01 Cellstructurefunctionapi-222503660No ratings yet
- 2017-03-22 JC Stemskills Full Stem Ahead-ShortenedDocument18 pages2017-03-22 JC Stemskills Full Stem Ahead-Shortenedapi-222503660No ratings yet