Professional Documents
Culture Documents
Lung Lab
Lung Lab
Uploaded by
api-302696314Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lung Lab
Lung Lab
Uploaded by
api-302696314Copyright:
Available Formats
James 1
Shands James
4/29/2016
DOS 771 Clinical Practicum I
Planning Assignment (Lung)
Target organ(s) or tissue being treated: Lung tumor located near midline of right lung
Prescription: 200 cGy x 30 fractions = 6000 cGy
Contour all critical structures on the dataset. Place the isocenter in the center of the PTV (make
sure it isnt in air). Create a single AP field using the lowest photon energy in your clinic. Create
a block on the AP beam with a 1.5 cm margin around the PTV. From there, apply the following
changes (one at a time) to see how the changes affect the plan (copy and paste plans or create
separate trials for each change so you can look at all of them). Refer to Bentel, pp. 370-376 for
references:
*Organ at risk chart was developed for each plan variation and can be found in each plan section.
Patient was simulated supine with a headrest and knee cushion for patient comfort and
immobilization.
James 2
Plan 1: Create a beam directly opposed to the original beam (PA) (assign 50/50 weighting to
each beam)
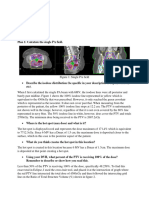
Figure 1: Plan 1 isodose lines from all planes. 100% line is yellow.
Organ at risk
Desired objective(s)
Achieved objective(s)
Spinal Cord1
Max Dose = 5000 cGy
Max Dose= 5652.8 cGy
Heart2
Mean Dose= 4000 cGy
Mean Dose = 995.6 cGy
V45<66%
V45 = 12.6%
V60<33%
Mean Dose = 3400 cGy2
V60 = 0%
Mean Dose = 1640.4 cGy
V35<50%
V35 = 26%
V50<40%
V5<65%
V50 = 10.4%
V5 = 92%
Left Lung(Contralateral)3
V5<60%
V5 = 0%
Total Lung (minus CTV)2
V20<37%
V20 = 39.7%
Esophagus
Right Lung(Ipsilateral)
Table 1: Dose statistics for Plan 1.
James 3
a. What does the dose distribution look like?
The first thing that I noticed about the isodose distribution is that it is not conformal
around the target. A large amount of the critical structures are being irradiated past
tolerance. The coverage for the PTV is also not very good.
b. Is the PTV covered entirely by the 95% isodose line?
No, the 95% line (Green) does not cover the entire PTV. The isodose line that does cover
the whole PTV is the 80% line (baby blue).
c. Where is the region of maximum dose (hot spot)? What is it?
The hotspot is located posteriorly on the patients back before the rib cage. The hotspot is
7129.4 cGy which is a 118.8% hotspot. This is located more posteriorly because the PA
beam has more tissue to penetrate before reaching the target.
Plan 2: Increase the beam energy for each field to the highest photon energy available.
Figure 2: Plan 2 isodose lines from all planes. 100% line is yellow. All beam energies
changed to 23 MV.
James 4
Organ at risk
Desired objective(s)
Achieved objective(s)
Spinal Cord1
Max Dose = 5000 cGy
Max Dose= 5765.9 cGy
Heart2
Mean Dose= 4000 cGy
Mean Dose = 998.1 cGy
V45<66%
V45 = 12.5%
V60<33%
Mean Dose = 3400 cGy2
V60 = 0%
Mean Dose = 1676.7 cGy
V35<50%
V35 = 26.2%
Right Lung(Ipsilateral)3
V50<40%
V5<65%
V50 = 14.5%
V5 = 93.4%
Left Lung(Contralateral)3
V5<60%
V5 = 0%
Total Lung (minus CTV)2
V20<37%
V20 = 39.9%
Esophagus
Table 2: Dose statistics for Plan 2.
a. What happened to the isodose lines when you increased the beam energy?
I switched to a 23 MV beam which caused the isodose lines shifted towards midline
which resulted in a lower hotspot and better PTV coverage. This shift is due to the 23
MV beam being more penetrating (dmax= 3.8 cm) compared to a 6 MV beam (dmax=
1.5cm).
b. Where is the region of maximum dose (hot spot)? Is it near the surface of
the patient? Why?
The hotspot is located posteriorly just before the start of the rib cage. The hotspot is
located here because there is more tissue that the PA beam has to penetrate than the
AP beam. So to deliver the prescription dose to the target more monitor units from the
PA beam are needed. However, the hotspot of plan 2 is located more anteriorly than
plan 1 because of the greater penetration of the 23 MV beam.
Plan 3: Adjust the weighting of the beams to try and decrease your hot spot.
James 5
Figure 3: Plan 3 isodose lines from all planes. 100% line is yellow. Weighting of each beam
was changed to reduce hotspot.
Organ at risk
Desired objective(s)
Achieved objective(s)
Spinal Cord1
Max Dose = 5000 cGy
Max Dose= 6061.2
Heart2
Mean Dose= 4000 cGy
Mean Dose = 711.3 cGy
V45<66%
V45 =5.8 %
V60<33%
Mean Dose = 3400 cGy2
V60 = 0%
Mean Dose = cGy
V35<50%
V35 = 6.7%
V50<40%
V5<65%
V50 = 0.9%
V5 = 91.5%
Left Lung(Contralateral)3
V5<60%
V5 =0 %
Total Lung (minus CTV)2
V20<37%
V20 = 38.3%
Esophagus
Right Lung(Ipsilateral)
Table 3:Dose statistics for Plan 3.
a. What ratio of beam weighting decreases the hot spot the most?
James 6
The beam weighting that reduced the hotspot the most was 54.5% from the AP beam and
45.5% from the PA beam. The hotspot was reduced to 105.9% from 109% (Plan 2) as
result of changing the weights of the beams.
b. How is the PTV coverage affected when you adjust the beam weights?
The PTV coverage of Plan 3 was not as good as Plan 2. In this case, the changing the
beam weighting reduced the coverage, but reduced the hotspot. This change in PTV
coverage is not what I expected to see when changing the beam weights.
Figure 4: DVH comparison of Plan 2 versus Plan 3.
James 7
Plan 4: Using the highest photon energy available, add in a 3rd beam to the plan (maybe a lateral
or oblique) and assign it a weight of 20%
Figure 5: Plan 1 isodose lines from all planes. An RPO beam was added to see the change in hotspot and PTV
coverage.
Organ at risk
Spinal Cord1
Desired objective(s)
Max Dose = 5000 cGy
Achieved objective(s)
Max Dose= 4354.9
Mean Dose= 4000 cGy
Mean Dose = 1167
V45<66%
V45 =12.9%
V60<33%
Mean Dose = 3400 cGy2
V60 = 0%
Mean Dose = 1723.4 cGy
V35<50%
V35 = 26.3%
V50<40%
V5<65%
V50 = 13.4%
V5 = 93.7%
Left Lung(Contralateral)3
V5<60%
V5 =0.21%
Total Lung (minus CTV)2
V20<37%
V20 = 40.2%
Heart2
Esophagus
Right Lung(Ipsilateral)
Table 4: Dose statistics for Plan 4.
James 8
a. When you add the third beam, try to avoid the cord (if it is being treated with the other
2 beams). How can you do that?
i. Adjust the gantry angle? I used an RPO beam at an angle of 210 degrees
which avoided an entrance and an exit on the cord. I also turned the
collimator so that the X jaw would better block the cord.
ii. Tighter blocked margin along the cord? On the side of the spinal cord
(X1), I changed the auto margin from 1.5 cm to a margin of 1 cm which
blocks the cord to a greater degree.
iii. Decrease the jaw alongside of the cord. After decreasing the margin
around the PTV, I adjusted the X1 jaw to fit snugly against the MLC to
further decrease the dose to the spinal cord by preventing leakage from the
MLC.
Figure 6: Beams eye view of RPO beam to improved blocking of the spinal cord.
James 9
b. Alter the weights of the fields and see how the isodose lines change in response
to the weighting. The lines conformed to the PTV better and the hotspot improved
as I adjusted the weight.
c. Would wedges help even out the dose distribution? If you think so, try inserting
one for at least one beam and watch how the isodose lines change. After
reviewing the locations of the hotspots, I dont think that a wedge would be helpful
in this case. Below is a picture of the AP beams eye view (BEV). The orange cloud
is the 105% location and does not fall to either side of the PTV.
Figure 7: BEV of AP beam showing areas of high dose(orange dose cloud) to determine
need for a wedge.
James 10
Which treatment plan covers the target the best? What is the hot spot for that plan?
Out of the 4 plans that were created during this project, Plan 4 had the best overall PTV
coverage and plan. Plan 2 was very close to the coverage that Plan 4 achieved however, Plan
4 was better overall. The hotspot for Plan 4 was 106.4% as opposed to 109% for Plan 2.
Did you achieve the OR constraints as listed above? List them in the table above.
None of the 4 plans achieved all of the dose constraints. The only plan to meet the spinal cord
constraint was Plan 4. The trade-off was higher lung, heart, and esophagus dose due to the
third beams entrance and exit locations.
What did you gain from this planning assignment?
This assignment helped me to see how adjusting the beam energies, angles, and weighting
can improve a plan. However, I noticed that there is a trade off when adjusting these factors.
For example, adjusting the beam weighting in Plan 3 decreased the hotspot, but the PTV
coverage got worse. Another thing that I noticed was the more beams that you add the more
conformal the isodose lines are and the hotspot decreases further.
What will you do differently next time?
The biggest change I would make would be in the beam arrangement. I would add more
beams to the plan so the dose will be more conformal. One drawback to the dataset that I
performed this assignment on was that the patient was simulated with their arms down
instead of in a wing board. Having the arms down means I cant have a lateral beam entrance
and limits the possible beam angles. Had the patient been simulated in a wing board a 5 field
beam arrangement may have been more appropriate and efficient at sparing the lung and
spinal cord. Having a 5 field beam arrangement will provide the opportunity of weighting the
fields in a different way with the goal being better conformity and a lower hotspot.
Another thing that I could change is the collimator rotations on all of the fields and not just
the RPO. I would angle the collimator to better block the spinal cord with the jaw. Also, I
would decrease the MLC margin along the side of the spinal cord to better preserve it.
Rotating the collimator so that the Y-jaw is perpendicular with the hotspot also provides the
possibility of using dynamic wedges that can improve coverage and the hotspot.
James 11
One last possibility of improving the plan is using a mixed energy beam arrangement. My
center only has 6MV and 23 MV on the machine that I planned on, but an energy somewhere
in between may be more appropriate. So using a combination of 6 MV of 23MV can give a
dose distribution of that is more suitable.
References
1. Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the
spinal cord. In J Radiat Oncol Biol Phys. 2010; 76 (3 Suppl): S42-9.
Http://10.1016/j.ijrobp.2009.04.095.
2. Videtic GMM, Woody NM. Handbook of Treatment Planning in Radiation Oncology. 2nd
ed. New York, NY: Demos Medical Publishing, LLC; 2015: 85-100.
3. Song CH, Pyo H, Moon SH, Kim TH, Kim DW, Cho KH. Treatment-related pneumonitis
and acute esophagitis in non-small-cell lung cancer patients treated with chemotherapy
and helical tomotherapy. Int J of Radiat Oncol Biol Phys. 2010; 78(3):651-8.
You might also like
- National Guidelines For The Use of Complementary Therapies in Supportive and Palliative CareDocument112 pagesNational Guidelines For The Use of Complementary Therapies in Supportive and Palliative Caredorothywyn50% (2)
- The Physics and Technology of Diagnostic Ultrasound: Study Guide (Second Edition)From EverandThe Physics and Technology of Diagnostic Ultrasound: Study Guide (Second Edition)No ratings yet
- Planning Project LungDocument5 pagesPlanning Project Lungapi-282901112No ratings yet
- Lung Clinical Lab AssignmentDocument13 pagesLung Clinical Lab Assignmentapi-528136686No ratings yet
- Lung LabDocument7 pagesLung Labapi-337264125No ratings yet
- Pelvis Lab 1 1Document13 pagesPelvis Lab 1 1api-632146706No ratings yet
- Lung Clinical Lab Assignment Write UpDocument16 pagesLung Clinical Lab Assignment Write Upapi-431720460No ratings yet
- Lung Lab Assignment 2Document19 pagesLung Lab Assignment 2api-631250296No ratings yet
- Lung Lab Ws 1Document14 pagesLung Lab Ws 1api-692310570No ratings yet
- Lung Clinical Lab Assignment2Document10 pagesLung Clinical Lab Assignment2api-425716959No ratings yet
- Lung Lab Dos 771Document18 pagesLung Lab Dos 771api-632146706No ratings yet
- Lung Clinical Lab AssignmentDocument8 pagesLung Clinical Lab Assignmentapi-432489466No ratings yet
- Lung Lab AssignmentdylDocument13 pagesLung Lab Assignmentdylapi-527669043No ratings yet
- LunglabpdfDocument21 pagesLunglabpdfapi-632827798No ratings yet
- Lung LabDocument20 pagesLung Labapi-691862313No ratings yet
- Pelvis Clinical Lab AssignmentDocument13 pagesPelvis Clinical Lab Assignmentapi-528136686No ratings yet
- Lung LabDocument9 pagesLung Labapi-712312990No ratings yet
- Lung LabDocument21 pagesLung Labapi-710804215No ratings yet
- Lung Planning AssignmentDocument5 pagesLung Planning Assignmentapi-265264098No ratings yet
- Lung LabweeblyDocument7 pagesLung Labweeblyapi-312374730No ratings yet
- 3 FD RectumDocument13 pages3 FD Rectumapi-279520503No ratings yet
- Lung Planning LabDocument9 pagesLung Planning Labapi-691277740No ratings yet
- Planning Assignment (3 Field Rectum)Document14 pagesPlanning Assignment (3 Field Rectum)api-280277788No ratings yet
- Lung Lab-MunaDocument26 pagesLung Lab-Munaapi-693187588No ratings yet
- LungDocument3 pagesLungapi-247959633No ratings yet
- LunglabDocument15 pagesLunglabapi-644802109No ratings yet
- Lung AssignmentDocument7 pagesLung Assignmentapi-280277788No ratings yet
- Lung LabDocument11 pagesLung Labapi-692616289No ratings yet
- Lung Lab RaverDocument9 pagesLung Lab Raverapi-635923017No ratings yet
- Lung Clinical Lab AssignmentDocument10 pagesLung Clinical Lab Assignmentapi-429099049No ratings yet
- Lunglab Josephspencer 1Document15 pagesLunglab Josephspencer 1api-633248237No ratings yet
- PelvislabpdfDocument17 pagesPelvislabpdfapi-632827798No ratings yet
- Lung Lab-Ht 2Document17 pagesLung Lab-Ht 2api-642692363No ratings yet
- Lung Lab Complete 1Document16 pagesLung Lab Complete 1api-688315037No ratings yet
- Lung Lab Responses WeeblyDocument15 pagesLung Lab Responses Weeblyapi-696520673No ratings yet
- Pelvis LabDocument19 pagesPelvis Labbraith7811No ratings yet
- Clinical Lung LabDocument17 pagesClinical Lung Labapi-632526087No ratings yet
- Lung Clinical Lab AssignmentDocument8 pagesLung Clinical Lab Assignmentapi-534702185No ratings yet
- Pelvis Lab HT 2Document15 pagesPelvis Lab HT 2api-642692363No ratings yet
- Final Lung LabDocument21 pagesFinal Lung LabpaulaNo ratings yet
- Lung Lab No 1Document11 pagesLung Lab No 1api-692147011No ratings yet
- Pelvis Clinical LabDocument27 pagesPelvis Clinical Labapi-710804215No ratings yet
- Clinical Practicum II Parotid Planning AssignmentDocument13 pagesClinical Practicum II Parotid Planning Assignmentapi-299270003No ratings yet
- Lung LabDocument13 pagesLung Labapi-692385376No ratings yet
- Lung Lab Instructions 1 1Document12 pagesLung Lab Instructions 1 1api-645453685No ratings yet
- 3d LungDocument7 pages3d Lungapi-352698073No ratings yet
- Pelvis Lab AllisonwrightDocument7 pagesPelvis Lab Allisonwrightapi-632741700No ratings yet
- Lung Lab AllisonwrightDocument13 pagesLung Lab Allisonwrightapi-632741700No ratings yet
- Pelvis Lab 1Document8 pagesPelvis Lab 1api-692147011No ratings yet
- Pelvis Lab 2021new-1 6Document6 pagesPelvis Lab 2021new-1 6api-635186395No ratings yet
- Lung Lab Instructions 1Document12 pagesLung Lab Instructions 1api-635186395No ratings yet
- Lung Lab SDocument13 pagesLung Lab Sapi-694839765No ratings yet
- Pelvis Lab AssignmentDocument13 pagesPelvis Lab Assignmentapi-631250296No ratings yet
- Peter Hirschi Spring 2015: Planning Assignment (Lung)Document8 pagesPeter Hirschi Spring 2015: Planning Assignment (Lung)api-279520503No ratings yet
- Lung Clinical Lab AssignmentDocument6 pagesLung Clinical Lab Assignmentapi-373874340No ratings yet
- Breast Plan Discussion Write UpDocument25 pagesBreast Plan Discussion Write Upapi-632827798No ratings yet
- Dans Lung Lab AssignmentDocument8 pagesDans Lung Lab Assignmentapi-395602816No ratings yet
- Pelvis Lab 2021-1 2Document11 pagesPelvis Lab 2021-1 2api-642376263No ratings yet
- LungDocument8 pagesLungapi-267335639No ratings yet
- Lung Lab Final 1 1Document11 pagesLung Lab Final 1 1api-428652649No ratings yet
- Pelvis LabDocument5 pagesPelvis Labapi-691544828No ratings yet
- Dos 542 Qa TableDocument28 pagesDos 542 Qa Tableapi-302696314No ratings yet
- Sample BudgetDocument6 pagesSample Budgetapi-302696314No ratings yet
- Imrtvvmat ProstateDocument19 pagesImrtvvmat Prostateapi-302696314No ratings yet
- Neck PP For WeeblyDocument4 pagesNeck PP For Weeblyapi-302696314No ratings yet
- Treatment Planning ProjectDocument12 pagesTreatment Planning Projectapi-302696314No ratings yet
- Dos711statisticsactiviy Group1Document4 pagesDos711statisticsactiviy Group1api-302696314No ratings yet
- Thorax PP For WeeblyDocument4 pagesThorax PP For Weeblyapi-302696314No ratings yet
- Combank NotesDocument7 pagesCombank NotesVee MendNo ratings yet
- SMI - Cardiovascular - New PDFDocument2 pagesSMI - Cardiovascular - New PDFprathibha asok kumarNo ratings yet
- Scratch Collapse TestDocument7 pagesScratch Collapse TestMikeunoeNo ratings yet
- Ao 2018-001Document31 pagesAo 2018-001MedFlight Ambulance corpNo ratings yet
- My Resume Luis SanchezDocument2 pagesMy Resume Luis Sanchezapi-242986404No ratings yet
- Complications Failures and Maintainence of Dental Implant 160218154535Document45 pagesComplications Failures and Maintainence of Dental Implant 160218154535DrIbrahimShaikhNo ratings yet
- TORSECDocument8 pagesTORSECamr ahmedNo ratings yet
- Slides - 7 - Intracanal Medicaments & TemporizationDocument53 pagesSlides - 7 - Intracanal Medicaments & Temporizationبراءة أحمد السلاماتNo ratings yet
- Indian Chilhood CirrhosisDocument28 pagesIndian Chilhood CirrhosisBelbi MolNo ratings yet
- Least Used ProtocolsDocument3 pagesLeast Used ProtocolsJohn Britto0% (1)
- Diagnosis and Treatment of Cyclothymia - The "Primacy" of TemperamentDocument8 pagesDiagnosis and Treatment of Cyclothymia - The "Primacy" of Temperamentkazzman56No ratings yet
- Contoh PPT DrowningDocument54 pagesContoh PPT DrowningIzzati SaidahNo ratings yet
- Detail 26223 2185634Document6 pagesDetail 26223 2185634api-487456788No ratings yet
- Rabies: 1 and 3 PeopleDocument6 pagesRabies: 1 and 3 PeopleDumapis RichardNo ratings yet
- Bos Specialty Assignment Cardiology-AmbroseDocument11 pagesBos Specialty Assignment Cardiology-Ambroseapi-396965096No ratings yet
- Costin Silviu: Lab TechnicianDocument1 pageCostin Silviu: Lab TechnicianSilvio DTNo ratings yet
- MyelopathyDocument10 pagesMyelopathyPutri WulandariNo ratings yet
- Hemodialysis Power PointDocument40 pagesHemodialysis Power Pointahmad_taher_9100% (1)
- Antiarritmicos PPT FinalDocument81 pagesAntiarritmicos PPT FinalCarolina González RiveraNo ratings yet
- Nursing Journal CTDocument3 pagesNursing Journal CTCharlene Jacobe CornistaNo ratings yet
- Kidney, Ureter, BladderDocument12 pagesKidney, Ureter, Bladdersarguss14100% (1)
- Neuro CaseDocument4 pagesNeuro CaseHannah MiaNo ratings yet
- Drug Therapy Assessment Worksheet (Dtaw) : 1. A Problem ExistsDocument6 pagesDrug Therapy Assessment Worksheet (Dtaw) : 1. A Problem ExistsputriNo ratings yet
- Updated Resume September 2019Document4 pagesUpdated Resume September 2019api-329713416No ratings yet
- ECG Workshop 2006Document15 pagesECG Workshop 2006Eggi ErlanggaNo ratings yet
- Antibiotics in Endodontics: A ReviewDocument16 pagesAntibiotics in Endodontics: A ReviewHisham HameedNo ratings yet
- Common Drugs and Antidotes: Substance AntidoteDocument2 pagesCommon Drugs and Antidotes: Substance AntidoteJo Ellie MarfilNo ratings yet
- Shouldice Hospital LTD: Group Case presentation-NMP25Document9 pagesShouldice Hospital LTD: Group Case presentation-NMP25Krishna TiwariNo ratings yet
- Slide RCA 23.3.2023Document32 pagesSlide RCA 23.3.2023Shah RizalNo ratings yet