Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
6 viewsTips On Writing The Lab Report
Tips On Writing The Lab Report
Uploaded by
baller0417Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF or read online from Scribd
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5835)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Uncle John'sDocument33 pagesUncle John'sballer041750% (2)
- Write Up TutorialDocument22 pagesWrite Up Tutorialballer0417100% (1)
- PortersDocument1 pagePortersballer0417No ratings yet
- Cranial Nerves and BranchesDocument5 pagesCranial Nerves and Branchesballer0417No ratings yet
Tips On Writing The Lab Report
Tips On Writing The Lab Report
Uploaded by
baller04170 ratings0% found this document useful (0 votes)
6 views4 pagesOriginal Title
Tips on Writing the Lab Report
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF or read online from Scribd
Download as pdf
0 ratings0% found this document useful (0 votes)
6 views4 pagesTips On Writing The Lab Report
Tips On Writing The Lab Report
Uploaded by
baller0417Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF or read online from Scribd
Download as pdf
You are on page 1of 4
le te
=
=
2
= A. Table of Properties
= This portion of the report must contain relevant properties of the reagents that
: you will be using in a particular experiment. For example, in the Recrystallization of
.
= Benzoie Acid, take note of the structure of benzoic acid, its boiling point, its melting
= point, its solubility at 100°C and at 0°C in a particular solvent, its physical appearance,
and safety precautions, if there are any. A particular compound has several properties.
‘Thus, you only need to include those properties that are important in a particular activity.
For instance, the following things are expected for benzoic acid in the aforementioned
= experiment:
- Y You want to know the structure of the compound because from its
4 structure, you will be able to deduce the kind of interactions that will be
a present between benzoic acid and the solvent, which in this case is water.
“| Y You want to know the boiling point to check ifthe compound evaporates
: ata particular temperate.
Y You want to know its melting point for the melting point determination
and for the recrystallization process.
¥ You want 10 know its solubility at 100°C and at 0°C in a particular
solvent to know if, indeed, your solvent is suitable for the
= recrystallization of the compound. Also, there are prelab/postiab
= questions that require knowledge of certain solubility constants.
7 Y You want to know the description (physical properties - color, physical
: state, odor) of the erystals of pure benzoic acid so that you can compare
your product with the pure one. At least, you already have a rough
Judgment on whether you gota pure product or not.
In the extraction process, you want to know the densities and solubilities
‘af compounds because the values will tell in which layers compounds are
expected to mix or be dissolved.
¥ In cases when the IR spectrum of the sample will be obtained, list down
beforehand the specific wavelengths at which the fimetional groups of
the compound will absorb.
=
=
3
=
=
=
©
Finally, safety precautions are self-explanatory.
B. Reaction
‘The reaction must be presented in a balanced chemical equation. This will help
you in calculating for your theoretical yild later on, This pat is very important because
the equation already summarizes what you are about todo inthe laboratory.
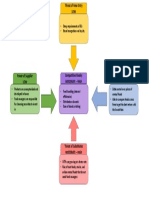
©. Flowchart
‘This portion is usually taken for granted since most students just copy from what
is written in the procedure, The flowchart of the experiment's procedure will guide you
a5 you perform the experiment. Students who do not have an organized flowchart of the
things to be done on the day of the experiment are usually the ones who encounter
‘accidents and blunders in the laboratory.
D. Prelaboratory Questions
Questions before the laboratory session are meant fo test your understanding and
prepare you on the experiment that you are about to do. These questions must be
answered thoroughly and explained in your own words, Highfalutin words and chemical
terms are not impressive unless they are thoroughly explained,
E. Data
ata obtained from the experiment should be recorded immediately in your
laboratory notebook. Relevant daa like weight of crude sample and weight of product,
te ae the ones that are expected in this part. Write down the information as reported by
the instrument. Example, if the balance reports data up to four decimal places, then
2.0000 g is not 2 g, More importantly, do not forget the units. It is also. your
‘esponsibility tobe consistent in the numberof significant figures, Not all balances in the
laboratory report data inthe same mumber of significant figures, Finally, in dealing with
instruments do not forget to write down in your laboratory notebook the model of the
instrument as well asthe parameters under which the measurement is taken,
F. Observations
u
Sha a be bb a ebb 0 eV Me |
(Leon
©
From the very stat of your experiment, immediately write down your
‘observations ie, description (physical properties) of your erude sample. As you go along.
‘with the experiment, ake note of what happened, if there are any separations — describe
the two layers ofthe residue and the filtrate, and describe your final prodc. You should
‘be creative inthis part. Most ofthe time, your teacher will not be 100% present as you do
the experiment, Thus, i is important that you take down everything so that other people
will know what you did in the experiment. Rule of thumb: Be concrete in writing
descriptions.
G, Results
Results must be calculated correctly and reported in the correct number of
significant figures, If the experiment only involves isolation or purification of
compound, the percentage recovery, which is just the ratio of the amount of product
‘obtained to the amount of the starting material multiplied by 100, should be reported. In
synthesis experiments, the theoretical, actual, and percentage yields are important. The
theoretical yield is the maximum product that the reaction can yield and is calculated
{rom the limiting reagent, whieh is ane ofthe starting materials present in molar deficit
(On the other hand, the actual yield is the amount that is obtained experimentally. The
percentage yleld is celewated asthe actual yield over the theoretical, times 100,
Yoyield = actual yield x 100
theoretical yield
H. Discussion
‘To be able to write a good discussion, make an outline of what you are about to
write first. I is even advisable that you discuss among your classmates what you did in
the laboratory session — the theory behind the experiment, the things that fed you to
produce such quality of product, ete, There are also a lot of references that are available
in the [brary that could enlighten you in your attempt fo understand the theory behind the
Jaboratory experiment, You ean cite these references but never plagiarize, Your teacher
will definitely know if you just copied the whole statement from a book or a journal
It is important that you will be able to link your research to whet you did in the
laboratory. What do you think are the reasons why you got this yield? What went wrong
and what should be done, instead? Can you say that your product is pure even ifyou did
not analyze i for its purity? Why? Ut is in this part that you will realize how indispensable
‘your lab notebook is, especially if you wrote down inthe observations section the things
that happened as you performed the experiment.
‘Remember that ths portion does not have to be lengthy. As Shakespeare wrote in
Hamlet, “brevity isthe soul of wit" It is not necessary to write a five-page single-spaced
discussion to get a good grade, A page-long discussion is already enough as long as you
hhave already explained the relevant details in the experiment
‘And do not forget to waite down your references. Your teacher may want to read
those references for leisure purposes.
G. Postlaboratory Questions
‘These are questions to supplement your understanding of the experiment and of
the theory behind it.
Ifyou are to submit @ handwritten report
‘make sure that your handwriting is respectably legible. ©
3B
=
=
=
=
=
e
=
=
=
=
=
=
;
5
5
=
=
=
q
5
=
=
=
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5835)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Uncle John'sDocument33 pagesUncle John'sballer041750% (2)
- Write Up TutorialDocument22 pagesWrite Up Tutorialballer0417100% (1)
- PortersDocument1 pagePortersballer0417No ratings yet
- Cranial Nerves and BranchesDocument5 pagesCranial Nerves and Branchesballer0417No ratings yet