Professional Documents
Culture Documents
PV NRT: Chemistry Paper 2 Answers
PV NRT: Chemistry Paper 2 Answers
Uploaded by
Harmony TanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
PV NRT: Chemistry Paper 2 Answers
PV NRT: Chemistry Paper 2 Answers
Uploaded by
Harmony TanCopyright:
Available Formats
Final Examination 2011 CHEMISTRY Paper 2 Answers Q 1(a) Answer pV = nRT
pV nT 101 .3x10 3 x 22 .41 x10 3 = 1x 273 .0 R=
Marks
1 1 2 1
(b) (i) (ii)
= 8.316 J K-1 mol-1 The presence of intermolecular forces of attraction between the chlorine molecules Under high temperatures and low pressures At high temperatures, the molecules have more kinetic energy At low pressures, the molecules are further apart Intermolecular forces become less significant
1 1 1 1 4
(c) (i)
Number of moles of X =
800 22 .41 x10 3
= 0.0357 mol Mr of X =
1.00 0.0375
= 28.01 C2H4
1 1
2 1 10
Q 2 (a) (i) (ii)
Answer Hunds rule 3d 4s
Mark 1 1
(iii) (iv)
2 Decrease in the electron-electron repulsion
1 1 4
(b) (i) 2H2O2 2H2O + O2 (ii)
Energy uncatalysed uncatalysed
Reaction pathway
Same H Labeled curves One hump Or two humps
1 1 1 1 1 2 3
(iii)
Colourless to brown // Brown to colourless Gas evolved / efferversence
10
Q 3(a) (i)
Answer Ground state: Excited state: 2s 2p
Mark
1 1 1 1 1 1 4 2
2s 2p 3 (ii) - Carbon atom undergoes sp hybridisation - One 2s orbital and the three 2p orbitals hybridise to form four new equivalent sp3 hybrids - Each hybrid orbital overlaps with the s orbital of the hydrogen atom to form bond - The shape of the molecule is tetrahedral 3 (b) (i) N H
107o
1 H H 1 1 1 3 10
- Nitrogen is more electronegative than phosphorus (ii) - Thus the bond angle H N H is larger than the bond angle H P H - The bonding electron pairs are closer to the nitrogen atom and causes more repulsion
Q 5 (a)
Answer PCO = PH2 = 183 kPa Kp = PH2 x PCO PH2O = ( 183kPa ) x (183 kPa) (90.0 kPa) Or
Mark 1
1 1 1 1
(b)
= 372 kPa Acids : C6H5OH CH3COOH (ii) CH3COOH is stronger acid than C6H5OH The position of equilibrium is very much to the right because of the very large Kc value. Or The proportions / concentrations of C6H5OH and CH3COO- are very much greater than C6H5O- and CH3COOH
1 2 1 1 1 1 3 10
(c) (i) Pb (aq) + CrO4 (aq) PbCrO4(s) (ii) Ksp = [Pb2+][CrO42-] [Pb2+][CrO42-] = 1.69 x 10-14 [Pb2+]2 = 1.69 x 10-14 Solubility = [Pb2+] = 1.3 x 10-7 mol dm-3
2+
2-
Q Answer 6 (a) Group 8 and Period 4 Physical properties 1. High melting point 2. Good electrical conductor Any one of the above Chemical properties 1. Form complex ions 2. Coloured compounds 3. Exhibit multiple oxidation states. 4. Act as catalyst Any one of the above (b) (i) A phase diagram is a graphical representation of the conditions of temperature and pressure at which a substance can exist as a single phase or as two or more phases in equilibrium. ii)
Mark 1+1
1 1
1. Correct shape 2. Correct labels for Pressure and Temperature 3. Correct label for triple point and critical point Below triple point Water vapour to ice/solid and then ice/solid to liquid Above triple point Water vapour to liquid
1 1 1 3
1 1 2
1. correct shape and labels
(iv) 1. Decreasing pressure at constant temperature below triple. 2. Increasing temperature at constant pressure below triple point 3 & 4. Show correct pathways.
1 1 1+1
5 15
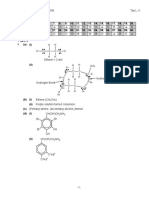
Q 7(a)
Answer The number of electrons in the outermost shell in boron atom B - 3e 4H - 4e (-) charge - 1e Total = 8e The shape of the molecule is tetrahedral
H
Mark
1 _ 1+1
109.5o
B
H
H
The number of electrons in the outermost shell in sulphur atom S - 6e 6F - 6e Total = 12 e 1 The shape of the molecule is octahedral
F F 90o F F
S
F F
1+1 1
(b)
1. CCl4 molecule is non-polar 2. Chlorine atom is more electronegative than carbon atom. The pairs of bonding covalent electrons are attracted more to the chlorine atom. Or Chlorine atoms thus possess a partial negative charge while carbon atom possesses a partial positive charge. 3. However, because the shape of the molecule is symmetrical, 4. There is a cancellation of dipole moment / the net dipole moment is zero.
1 1 4
(c) (i)
- Equilibrium shifts to left at high pressure - because position of equilibrium moves to favour fewer moles (of gas) - At high temperature reaction yield is low (or vice versa) - At low temperature reaction is slow (or vice versa) 1 - Therefore use a balance (or compromise) between rate and yield 1
1 1 1 1 1
15
8. 2
3 2
3
(iv) phenolphthalein
15 marks
You might also like
- Chem Student Book 2 AnswersDocument68 pagesChem Student Book 2 AnswersMeeran Hassan96% (27)
- 2011 H2 Chemistry Paper 3 Suggested SolutionsDocument7 pages2011 H2 Chemistry Paper 3 Suggested SolutionsLee Jun Hui0% (1)
- IAL Chemistry SB2 Answers Topic11Document7 pagesIAL Chemistry SB2 Answers Topic11salmaNo ratings yet
- 2010 A Level H2 P3 Suggested AnswersDocument10 pages2010 A Level H2 P3 Suggested AnswersMichelle LimNo ratings yet
- 2011 H2 Chemistry Paper 1 Suggested SolutionsDocument18 pages2011 H2 Chemistry Paper 1 Suggested SolutionsLee Jun HuiNo ratings yet
- Sample PaperDocument9 pagesSample PaperPc xoixaNo ratings yet
- CBSE Class 11 Chemistry Sample Paper Set 1 Solution PDFDocument9 pagesCBSE Class 11 Chemistry Sample Paper Set 1 Solution PDFBalajiNo ratings yet
- Class 11Document6 pagesClass 11Anitha SathiaseelanNo ratings yet
- Chem 11Document5 pagesChem 11Anitha SathiaseelanNo ratings yet
- Mass of The Compound 0.3780 G Mass of Silver Chloride 0.5740 GDocument10 pagesMass of The Compound 0.3780 G Mass of Silver Chloride 0.5740 Gnivrutiverma1234No ratings yet
- NSEC 2022 Question Paper With SolutionsDocument32 pagesNSEC 2022 Question Paper With SolutionsmaanasbaniNo ratings yet
- Fiitjee: Solutions To AIEEE-2007-CHEMISTRY Paper Code (O) - 1Document9 pagesFiitjee: Solutions To AIEEE-2007-CHEMISTRY Paper Code (O) - 1Lokesh KumarNo ratings yet
- 11che02 QPDocument5 pages11che02 QPPriyanshu BadhiaNo ratings yet
- Ri h2 Chem p2 AnswersDocument7 pagesRi h2 Chem p2 Answersdragon slayerNo ratings yet
- Eamcet 2008 EnggDocument15 pagesEamcet 2008 EnggjanmanchiNo ratings yet
- Model Answers To Specimen Question Paper For Xii Isc Board Examination 2012Document18 pagesModel Answers To Specimen Question Paper For Xii Isc Board Examination 2012santhosh1995No ratings yet
- Class XI Chem SAMPLEDocument4 pagesClass XI Chem SAMPLEFIITJEE DPSNo ratings yet
- MS Xi ChemistryDocument5 pagesMS Xi ChemistryMayankNo ratings yet
- Nov 2008Document13 pagesNov 2008dharshanaabNo ratings yet
- Asm1 Chemistry 253147Document6 pagesAsm1 Chemistry 253147deek_jNo ratings yet
- Contest 17Document3 pagesContest 17bakosua141No ratings yet
- VJC 2007Document14 pagesVJC 2007sswee_1No ratings yet
- 108下試題 (含解答)Document6 pages108下試題 (含解答)wanyun345No ratings yet
- Chemistry - Sample Question Paper - 9Document6 pagesChemistry - Sample Question Paper - 9Mohd AdilNo ratings yet
- Simulado - AnswerDocument10 pagesSimulado - AnswerMatheus MarquesNo ratings yet
- 2012 HCI H2 Chemistry Paper 3 Answers For Other JCsDocument9 pages2012 HCI H2 Chemistry Paper 3 Answers For Other JCsKen JiaNo ratings yet
- Compartment 2 Chem QPDocument5 pagesCompartment 2 Chem QPAAKASH BHATTNo ratings yet
- AIPMT 2015 Sample PaperDocument26 pagesAIPMT 2015 Sample PaperFirdosh Khan100% (3)
- Chemistry Common Answer KeyDocument10 pagesChemistry Common Answer KeyiskypiskybruhNo ratings yet
- CHEM 1000 Mid-Year Exam December 2002: Part A. 60 Marks. Answer Each Question (5 Marks Each)Document7 pagesCHEM 1000 Mid-Year Exam December 2002: Part A. 60 Marks. Answer Each Question (5 Marks Each)Geleni Shalaine BelloNo ratings yet
- Chemistry: Q.1. A Solution Containing 2.675 G of CoclDocument14 pagesChemistry: Q.1. A Solution Containing 2.675 G of CoclArun Kumar Arun KumarNo ratings yet
- QP Chem - XI - 2019-20 11Document5 pagesQP Chem - XI - 2019-20 11Lawrence GaikwadNo ratings yet
- 11-Chemistry-A1-Annual Exam 2023-24Document8 pages11-Chemistry-A1-Annual Exam 2023-24harshitsharmasportsNo ratings yet
- SAMPLE PAPER - FinalTerm - GR11 - 2023-24Document8 pagesSAMPLE PAPER - FinalTerm - GR11 - 2023-24collect3.141No ratings yet
- Jee Main 2014 KeyDocument14 pagesJee Main 2014 KeyutkarshrodgeNo ratings yet
- Final Exam Study GuideDocument15 pagesFinal Exam Study Guidekramark808No ratings yet
- CHEM 101 Past Questions With Answers by TihboiDocument10 pagesCHEM 101 Past Questions With Answers by TihboiDhar MieNo ratings yet
- JEE Main Solutions 2016 Aakash Code FDocument21 pagesJEE Main Solutions 2016 Aakash Code Famit_idea1No ratings yet
- J1 Promos 2015 AnswersDocument14 pagesJ1 Promos 2015 AnswersaliciaNo ratings yet
- HCI 2021 Prelim Paper 1 SolutionsDocument18 pagesHCI 2021 Prelim Paper 1 Solutions4A730RudhreshNo ratings yet
- MCQs For Class XII ChemistryDocument29 pagesMCQs For Class XII Chemistryjkc collegeNo ratings yet
- PT 2 Chemistry Paper (2023-24)Document3 pagesPT 2 Chemistry Paper (2023-24)amoeba220106No ratings yet
- Class XII - All India Chemistry - Set-2: Alkyl Halide Sodium Alkoxide EtherDocument4 pagesClass XII - All India Chemistry - Set-2: Alkyl Halide Sodium Alkoxide EtherShashank ShekharNo ratings yet
- (Done Edu - Joshuatly.com) Kedah STPM Trial 2010 Chemistry (W Ans) (33E48B52)Document0 pages(Done Edu - Joshuatly.com) Kedah STPM Trial 2010 Chemistry (W Ans) (33E48B52)BlaireNo ratings yet
- كيمياء طبية3Document6 pagesكيمياء طبية3هيثم الساعديNo ratings yet
- 1991 AL Chem MSDocument20 pages1991 AL Chem MSrelaxmore123No ratings yet
- Chem EassessDocument13 pagesChem Eassesswhitebrenda30No ratings yet
- Downloading - Viswa Niketan Secondary School (11 & 12)Document32 pagesDownloading - Viswa Niketan Secondary School (11 & 12)Sāŕőj ÝáđåvNo ratings yet
- Chem Halfyrly 2020Document6 pagesChem Halfyrly 2020ShraddhaNo ratings yet
- MCAT Chemistry TestDocument6 pagesMCAT Chemistry TestSehbaz KhanNo ratings yet
- Nanyang Technological University Singapore Entrance Examination CHEMISTRY (Sample) InstructionsDocument8 pagesNanyang Technological University Singapore Entrance Examination CHEMISTRY (Sample) InstructionsAriny Lastarya PutriNo ratings yet
- KEAM 2014 Medical Solution - Physics and ChemistryDocument7 pagesKEAM 2014 Medical Solution - Physics and ChemistryAnweshaBose0% (1)
- Guess Paper Chemistry XI - SECTION CDocument6 pagesGuess Paper Chemistry XI - SECTION Csabakhanmanu1234No ratings yet
- Code 0: Iit - Jee (2011) Paper Ii Question & SolutionsDocument25 pagesCode 0: Iit - Jee (2011) Paper Ii Question & SolutionskapilNo ratings yet
- HKALE Chemistry 2001 Marking SchemeDocument7 pagesHKALE Chemistry 2001 Marking SchemeHon KwanNo ratings yet
- Xi - ChemistryDocument4 pagesXi - Chemistrybinodxyz0No ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)