Professional Documents
Culture Documents
4.6 Limited Pressure Cycle (Or Dual Cycle)
4.6 Limited Pressure Cycle (Or Dual Cycle)
Uploaded by
yaser201xOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
4.6 Limited Pressure Cycle (Or Dual Cycle)
4.6 Limited Pressure Cycle (Or Dual Cycle)
Uploaded by
yaser201xCopyright:
Available Formats
Gas Power Cycles
Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.6 Limited Pressure Cycle (or Dual Cycle):
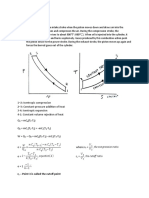
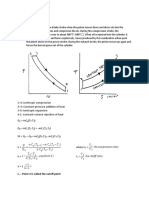
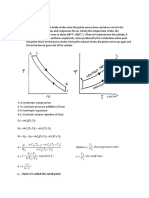
This cycle is also called as the dual cycle, which is shown in Fig.4.6. Here the heat addition occurs partly at constant volume and partly at constant pressure. This cycle is a closer approximation to the behavior of the actual Otto and Diesel engines because in the actual engines, the combustion process does not occur exactly at constant volume or at constant pressure but rather as in the dual cycle.
Process 1-2: Reversible adiabatic compression. Process 2-3: Constant volume heat addition. Process 3-4: Constant pressure heat addition. Process 4-5: Reversible adiabatic expansion. Process 5-1: Constant volume heat rejection.
2 5 1
Volume
Indian Institute of Technology Madras
Gas Power Cycles
Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Constant Volume Constant Pressure
4 3 2
Entropy
Fig.4.6. Dual cycle on p-v and T-s diagrams
Air Standard Efficiency:
Heat sup plied = m C v ( T3 - T2 ) + m Cp ( T4 - T3 )
Heat rejected = m C v ( T5 - T1 )
Net work done = m C v ( T3 - T2 ) + m Cp ( T4 - T3 ) - m CV ( T5 - T1 )
th =
m C v ( T3 - T2 ) + m Cp ( T4 - T3 ) - m C v ( T5 - T1 ) m C v ( T3 - T2 ) + m Cp ( T4 - T3 ) T5 - T1 ( T3 - T2 ) + ( T4 - T3 )
th = 1 -
Let,
P3 v v = rp ; 4 = rc ; 1 = r P2 v3 v2
T2 = T1 r - 1 T3 = T2 rp = T1 r - 1 rp T4 = T3 rc = T1 r - 1 rp rc
Indian Institute of Technology Madras
Gas Power Cycles
-1
Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
-1 -1
v T5 = 4 T4 v5
v v = 4. 2 v 2 v5
-1
r = c r
r T5 = T4 c r
= T1 rp rc
th = 1 -
{( T r
1
-1
rp - T1 r - 1 + T1 r - 1 rp rc - T1 r - 1 rp
T1 rp rc - T1
)}
( rp rc - 1) = 1{( rp r - 1 - r - 1 ) + ( rp rc r - 1 - rp r - 1 )}
th rp rc - 1 1 1 - -1 r rp - 1 + rp ( rc - 1)
From the above equation, it is observed that, a value of rp > 1 results in an increased efficiency for a given value of rc and . Thus the efficiency of the dual cycle lies between that of the Otto cycle and the Diesel cycle having the same compression ratio.
Mean Effective Pressure:
mep =
Workdone Displacement volume
m C v ( T3 - T2 ) + m Cp ( T4 - T3 ) - m C v ( T5 - T1 ) v1 - v 2
v1 - v 2 =
m C v ( - 1) T1 r - 1 p1 r
Indian Institute of Technology Madras
Gas Power Cycles
Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
mep =
T3 - T2 ( T4 - T3 ) T5 - T1 p1 r + T1 T1 ( r -1)( - 1) T1
p1 r r - 1 rp - 1 + r - 1 rp ( rc - 1) - rp rc - 1 r - 1)( - 1) ( p1 r r - 1 rp - 1 + rp ( rc - 1) - rp rc - 1 r - 1)( - 1) (
{ (
)}
{ {(
} (
)}
Indian Institute of Technology Madras
You might also like
- Ebook - Define App Requirements Within 20minsDocument11 pagesEbook - Define App Requirements Within 20minsAnonymous ChxaIdbchNo ratings yet
- BS en Iso 1833-12-2010Document12 pagesBS en Iso 1833-12-2010EmkFataAliraqNo ratings yet
- 9 Atkinson CycleDocument3 pages9 Atkinson CyclecaptainhassNo ratings yet
- 4.10 Brayton Cycle (Simple Gas Turbine Cycle) :: Fig.4.10. Brayton Cycle On P-V and T-S DiagramDocument3 pages4.10 Brayton Cycle (Simple Gas Turbine Cycle) :: Fig.4.10. Brayton Cycle On P-V and T-S DiagrammominjeelaniNo ratings yet
- 10 Lenoir CycleDocument2 pages10 Lenoir Cyclecaptainhass100% (1)
- Limited Pressure CycleDocument4 pagesLimited Pressure Cyclecasu19No ratings yet
- 11 - Dr. M. Atia - Gas Power Cycles-Dual CycleDocument20 pages11 - Dr. M. Atia - Gas Power Cycles-Dual CycleBahaa RaghebNo ratings yet
- Unit-Ii Diesel, Gas Turbine and Combined Cycle Power PlantsDocument71 pagesUnit-Ii Diesel, Gas Turbine and Combined Cycle Power Plantsrsankarganesh MECH-HICETNo ratings yet
- Gas Turbines Unit 3: Gas Turbines and Jet Propulsion: Classification of Gas Turbines, Analysis of Open Cycle GasDocument39 pagesGas Turbines Unit 3: Gas Turbines and Jet Propulsion: Classification of Gas Turbines, Analysis of Open Cycle GasJoseph MwansaNo ratings yet
- Applied Thermodynamics ME250: Submitted ToDocument12 pagesApplied Thermodynamics ME250: Submitted Tomad eye m00dyNo ratings yet
- B59TC Gas Power Plant NotesDocument23 pagesB59TC Gas Power Plant Noteshansdavid.aquino2004No ratings yet
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument5 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- Mod1 Te - Mod 2 Te - MergedDocument12 pagesMod1 Te - Mod 2 Te - Mergedsaikrishnaps31No ratings yet
- Ideal CyclesDocument2 pagesIdeal CyclesSofia OrjuelaNo ratings yet
- Brayton Cycle PDFDocument5 pagesBrayton Cycle PDFgirishgangari8845No ratings yet
- JEE Advanced Heat and Thermodynamics Important QuestionsDocument26 pagesJEE Advanced Heat and Thermodynamics Important QuestionsMayank VermaNo ratings yet
- CLS JEEAD-19-20 XI Phy Target-5 Level-2 Chapter-11 PDFDocument28 pagesCLS JEEAD-19-20 XI Phy Target-5 Level-2 Chapter-11 PDFVISHVESH GOYAL100% (1)
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument7 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- Principle of TurbomachineryDocument159 pagesPrinciple of TurbomachineryWalid MohammedNo ratings yet
- TDHT - 03 - Thermodynamic Cycles (Gas Steam) 1Document19 pagesTDHT - 03 - Thermodynamic Cycles (Gas Steam) 1djukwe keuzetienNo ratings yet
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument8 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- A Cooling Unit To Increase The Efficiency of Gas Turbine PlantDocument5 pagesA Cooling Unit To Increase The Efficiency of Gas Turbine PlantseventhsensegroupNo ratings yet
- Kmme Te101-3Document6 pagesKmme Te101-3ayla sözenNo ratings yet
- Final Exam MockDocument11 pagesFinal Exam Mockb8vfdrjff6No ratings yet
- Cycle CalculationsDocument6 pagesCycle CalculationsmudassarhussainNo ratings yet
- Air Cycle RefrigerationDocument10 pagesAir Cycle RefrigerationashisNo ratings yet
- Gas TurbineDocument7 pagesGas TurbineVishal BediNo ratings yet
- Gasturbine 1 160120155226Document52 pagesGasturbine 1 160120155226Muhammad Afif NaufalNo ratings yet
- Marine Diesel (WEEK - 3) Thermodynamics: Jurusan Teknik Sistem Perkapalan ITS SurabayaDocument38 pagesMarine Diesel (WEEK - 3) Thermodynamics: Jurusan Teknik Sistem Perkapalan ITS SurabayafaridNo ratings yet
- 3-TurboEngine Components CharacteristicsDocument10 pages3-TurboEngine Components CharacteristicsAbdou JirariNo ratings yet
- Otto CycleDocument5 pagesOtto CycleSaraju NandiNo ratings yet
- Mechanical Upto Ic Engine SpecifocationnDocument22 pagesMechanical Upto Ic Engine SpecifocationnlechuznimaNo ratings yet
- Otto Cycle, Fuels, Combustion PDFDocument39 pagesOtto Cycle, Fuels, Combustion PDFAchmad Rizal FirmansyahNo ratings yet
- Calculation of Time Required To Fill Tanks With Compressed GasDocument4 pagesCalculation of Time Required To Fill Tanks With Compressed Gas1940LaSalleNo ratings yet
- Prelim Ito Na PreDocument8 pagesPrelim Ito Na PreJethro Briza GaneloNo ratings yet
- Gas Power CyclesDocument140 pagesGas Power CyclesMohammed AlsirajNo ratings yet
- Tegar Ari Widianto 16306141051 Penyelesaian SoalDocument10 pagesTegar Ari Widianto 16306141051 Penyelesaian SoalTegar Ari WidiantoNo ratings yet
- 04 - Thermodynamic - Cycles - (Joule - B) PDFDocument8 pages04 - Thermodynamic - Cycles - (Joule - B) PDFAntonio Di FioreNo ratings yet
- Chap 23Document15 pagesChap 23민규No ratings yet
- Ideal Engine CycleDocument20 pagesIdeal Engine CycleMulugeta WoldeNo ratings yet
- Applied Thermodynamics and Heat Transfer - Unit IDocument37 pagesApplied Thermodynamics and Heat Transfer - Unit IArun ShankarNo ratings yet
- ICE - Lecture From MapuaDocument48 pagesICE - Lecture From MapuaMarcial Jr. MilitanteNo ratings yet
- Power Cycles 1 - 1 PDFDocument6 pagesPower Cycles 1 - 1 PDFclarkmaxNo ratings yet
- crs2 1Document2 pagescrs2 1Chayut NuntadusitNo ratings yet
- Thermo Cycles PDFDocument29 pagesThermo Cycles PDFWaseem Abbas AttariNo ratings yet
- 4 EngineCyclesAnalysisDocument9 pages4 EngineCyclesAnalysisNatthaphon NaosookNo ratings yet
- Compres Turbine Heating (Combustion) 2: g-1 G g-1 G g-1 GDocument7 pagesCompres Turbine Heating (Combustion) 2: g-1 G g-1 G g-1 GMrityunjay KrNo ratings yet
- Lecture 5 - Gas Turbine EnginesDocument11 pagesLecture 5 - Gas Turbine EnginesShailani HossainNo ratings yet
- Derive An Expression For Efficiency of Diesel CycleDocument3 pagesDerive An Expression For Efficiency of Diesel CycleME-Pratham JainNo ratings yet
- CHE3161 Semester1 2010 Solutions PDFDocument14 pagesCHE3161 Semester1 2010 Solutions PDFkumiristineNo ratings yet
- Internal Combustion EnginesDocument13 pagesInternal Combustion EnginesEunice Joy VillegasNo ratings yet
- Lecture Notes OnDocument200 pagesLecture Notes Onananth k r100% (3)
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- Commensurabilities among Lattices in PU (1,n). (AM-132), Volume 132From EverandCommensurabilities among Lattices in PU (1,n). (AM-132), Volume 132No ratings yet
- Q FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004Document5 pagesQ FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- Q FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004Document7 pagesQ FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- Lecture 14Document8 pagesLecture 14captainhassNo ratings yet
- d dT J Lq L dx dx: q=J (Π - Π) q=J (Π - Π)Document6 pagesd dT J Lq L dx dx: q=J (Π - Π) q=J (Π - Π)captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: F M F F FDocument5 pages2.57 Nano-to-Macro Transport Processes Fall 2004: F M F F FcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004 Guest Lecture by Prof. Mildred S. DresselhausDocument10 pages2.57 Nano-to-Macro Transport Processes Fall 2004 Guest Lecture by Prof. Mildred S. DresselhauscaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004:) N N N N (NDocument8 pages2.57 Nano-to-Macro Transport Processes Fall 2004:) N N N N (NcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: I T KXDocument8 pages2.57 Nano-to-Macro Transport Processes Fall 2004: I T KXcaptainhassNo ratings yet
- Lecture 18Document6 pagesLecture 18captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004 - Lecture 15 Guest Lecture by Prof. DresselhausDocument10 pages2.57 Nano-to-Macro Transport Processes Fall 2004 - Lecture 15 Guest Lecture by Prof. DresselhauscaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Ae Be CeDocument7 pages2.57 Nano-to-Macro Transport Processes Fall 2004: Ae Be CecaptainhassNo ratings yet
- N F T e U F T F TD D: 2.57 Nano-to-Macro Transport Processes Fall 2004Document6 pagesN F T e U F T F TD D: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document9 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- L-05 (GDR) (Et) ( (Ee) Nptel)Document11 pagesL-05 (GDR) (Et) ( (Ee) Nptel)nvnmnitNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Nlms 2Document7 pages2.57 Nano-to-Macro Transport Processes Fall 2004: Nlms 2captainhassNo ratings yet
- 2.57 Nano-To-Macro Transport Processes Fall 2004: Ikn A IkxDocument7 pages2.57 Nano-To-Macro Transport Processes Fall 2004: Ikn A IkxcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: DT Q K T Q DX Q (W/M K (W/M-K) Is Thermal ConductivityDocument5 pages2.57 Nano-to-Macro Transport Processes Fall 2004: DT Q K T Q DX Q (W/M K (W/M-K) Is Thermal ConductivitycaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: V L KK VKK N VDocument7 pages2.57 Nano-to-Macro Transport Processes Fall 2004: V L KK VKK N VcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: E HN MDocument10 pages2.57 Nano-to-Macro Transport Processes Fall 2004: E HN McaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document7 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Ja (j-1) A (j+1) ADocument9 pages2.57 Nano-to-Macro Transport Processes Fall 2004: Ja (j-1) A (j+1) AcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: A Wigner-Seitz Primitive Unit CellDocument6 pages2.57 Nano-to-Macro Transport Processes Fall 2004: A Wigner-Seitz Primitive Unit CellcaptainhassNo ratings yet
- L-44 (GDR) (Et) ( (Ee) Nptel)Document15 pagesL-44 (GDR) (Et) ( (Ee) Nptel)yashaswiyellapragadaNo ratings yet
- Problem Solving On D C Machines PDFDocument16 pagesProblem Solving On D C Machines PDFSelvaraj ParamasivanNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document8 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document7 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- Auto Transformer ModuleDocument10 pagesAuto Transformer ModuleIkhwanJackNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document6 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- L 07 (GDR) (Et) ( (Ee) Nptel)Document15 pagesL 07 (GDR) (Et) ( (Ee) Nptel)Aneurys DuranNo ratings yet
- Three-Phase Induction Motor: Version 2 EE IIT, KharagpurDocument8 pagesThree-Phase Induction Motor: Version 2 EE IIT, KharagpurHarsh PatelNo ratings yet
- Clojure Cheat Sheet A4 GreyDocument2 pagesClojure Cheat Sheet A4 GreyWFLXBjUcNo ratings yet
- Chapter #2 (Part 1)Document17 pagesChapter #2 (Part 1)Consiko leeNo ratings yet
- Cap 1201 PDFDocument54 pagesCap 1201 PDFDanita CatoNo ratings yet
- Nagothane Manufacturing Division Summer Internship ReportDocument5 pagesNagothane Manufacturing Division Summer Internship ReportMrudu PadteNo ratings yet
- CL7103 SystemTheoryquestionbankDocument11 pagesCL7103 SystemTheoryquestionbanksyed1188No ratings yet
- Water Storage Tanks For Sprinkler SystemsDocument4 pagesWater Storage Tanks For Sprinkler Systemsshanwen88100% (1)
- Strategy and Competitive Advantage Chapter 6Document7 pagesStrategy and Competitive Advantage Chapter 6Drishtee DevianeeNo ratings yet
- RR-0479-02 Novel Coronavirus (2019-nCoV) Real Time RT-PCR Kit-20200227 PDFDocument1 pageRR-0479-02 Novel Coronavirus (2019-nCoV) Real Time RT-PCR Kit-20200227 PDFwijaya adidarmaNo ratings yet
- Eureka Coin White Paper DraftDocument24 pagesEureka Coin White Paper DraftImdad AliNo ratings yet
- 3 Star Hotel - BaalbeckDocument19 pages3 Star Hotel - BaalbeckAkm EngidaNo ratings yet
- Oriental Happy Family Brochure PDFDocument10 pagesOriental Happy Family Brochure PDFRajat GuptaNo ratings yet
- WaterGEMS For ArcMap Sesion1Document38 pagesWaterGEMS For ArcMap Sesion1franklin2891100% (1)
- DVTK Storage SCP Emulator User ManualDocument20 pagesDVTK Storage SCP Emulator User Manualsegurah0% (1)
- Laws4112 (2014) Exam Notes (Uq)Document132 pagesLaws4112 (2014) Exam Notes (Uq)tc_bassNo ratings yet
- Groundnut@ShaliniDocument15 pagesGroundnut@Shalinishalini shuklaNo ratings yet
- Internal Analysis: Pertemuan KeDocument15 pagesInternal Analysis: Pertemuan Kekintan utamiNo ratings yet
- PQ 266 SL II P 03 Legal Framework Case Study PDFDocument3 pagesPQ 266 SL II P 03 Legal Framework Case Study PDFUshani CabralNo ratings yet
- Ebooks: Introducing Lippincott Williams & WilkinsDocument2 pagesEbooks: Introducing Lippincott Williams & WilkinsAndre SetiawanNo ratings yet
- JurisdictionDocument26 pagesJurisdictionSainathNo ratings yet
- Local Admin TrainingDocument68 pagesLocal Admin TrainingRavi YalalaNo ratings yet
- سيكادا 3301 - ويكيبيدياDocument5 pagesسيكادا 3301 - ويكيبيدياbtkmouradNo ratings yet
- Installation Manual: Middle Static Pressure Duct TypeDocument35 pagesInstallation Manual: Middle Static Pressure Duct TypecelsofortoulNo ratings yet
- Agnes vs. Republic of The Philippines (2015 Calauit Case)Document16 pagesAgnes vs. Republic of The Philippines (2015 Calauit Case)Jovhilmar EstoqueNo ratings yet
- Mule Core ComponentsDocument7 pagesMule Core ComponentsRameshChNo ratings yet
- JeffFurman Resume18Document3 pagesJeffFurman Resume18Prashant PrajapatiNo ratings yet
- Aircraft Design Project Designing A Competitor Fighter AircraftDocument45 pagesAircraft Design Project Designing A Competitor Fighter AircraftKarthick. GNo ratings yet
- WEG Sca06 Eco5 Eco6 Eco7 Modulo de Expansao de Comunicacao 10003320700 Guia de Instalacao Portugues BRDocument24 pagesWEG Sca06 Eco5 Eco6 Eco7 Modulo de Expansao de Comunicacao 10003320700 Guia de Instalacao Portugues BRWeverton CamposNo ratings yet
- Percona MySQL Support Services For Better Application PerformanceDocument6 pagesPercona MySQL Support Services For Better Application PerformanceGhanshyam BaviskarNo ratings yet