Professional Documents
Culture Documents
Sieve Plate Distillation Column - Lab Report
Sieve Plate Distillation Column - Lab Report
Uploaded by
Shrankhla NaryaCopyright:
Available Formats
You might also like
- Cement and Concrete Mineral Admixtures, by Tokyay M., 2016 PDFDocument325 pagesCement and Concrete Mineral Admixtures, by Tokyay M., 2016 PDFAngel Baraoed100% (3)
- Group21 Experiment11Document18 pagesGroup21 Experiment11kefiloe Letsie100% (1)
- Vapor in Air DiffusionDocument6 pagesVapor in Air DiffusionMeetNo ratings yet
- Lab6-Tubular Flow ReactorDocument11 pagesLab6-Tubular Flow ReactorNurtasha Atikah100% (1)
- CSTRDocument12 pagesCSTRsamueloNo ratings yet
- Solution:: Cosh Cosh 1 Cosh CoshDocument2 pagesSolution:: Cosh Cosh 1 Cosh CoshVaibhav GuptaNo ratings yet
- Greenwood Earnshaw Chemistry of The ElementsDocument4 pagesGreenwood Earnshaw Chemistry of The ElementsemreNo ratings yet
- Assignment (Air Pollution)Document7 pagesAssignment (Air Pollution)Durga Prasad Murmu0% (1)
- Sieve Plate Distillation ColumnDocument9 pagesSieve Plate Distillation ColumnAshish VermaNo ratings yet
- Separation Processes Lab ReportDocument15 pagesSeparation Processes Lab ReportArslanQureshi0% (1)
- Lab ManualDocument59 pagesLab ManualmarkNo ratings yet
- Exp-9 - Liquid Liquid Extraction in A Packed ColumnDocument5 pagesExp-9 - Liquid Liquid Extraction in A Packed ColumnSiddharth MohapatraNo ratings yet
- A Major Project Report On Design of Multicomponent Distillation Column by Approximate and Rigorous Method Using MatlabDocument51 pagesA Major Project Report On Design of Multicomponent Distillation Column by Approximate and Rigorous Method Using MatlabMasood HassanNo ratings yet
- CSTRDocument21 pagesCSTRirfan hilmanNo ratings yet
- Isothermal CSTR PDFDocument9 pagesIsothermal CSTR PDFprashant_cool_4_uNo ratings yet
- Sieve Plate DistillationDocument3 pagesSieve Plate DistillationRahul Pancholi67% (3)
- Theoretical Plates Calculation by McCabe-Thiele MethodDocument4 pagesTheoretical Plates Calculation by McCabe-Thiele Methodmohammad shoaibNo ratings yet
- CRE-II - Chapter-04 Fluid-Particle Systems - HKB 2.10.18Document42 pagesCRE-II - Chapter-04 Fluid-Particle Systems - HKB 2.10.18Ananya DaveNo ratings yet
- RI Vs Composition Methanol-Water MixtureDocument12 pagesRI Vs Composition Methanol-Water MixtureAnonymous VeJYFSMWLINo ratings yet
- Distillation Column DesignDocument20 pagesDistillation Column DesignSandeep Challa100% (1)
- CSTR 40LDocument16 pagesCSTR 40LhishamNo ratings yet
- Absorption and StrippingDocument60 pagesAbsorption and StrippingMyvizhi Somasundaram100% (2)
- Sieve Plate ColumnDocument14 pagesSieve Plate ColumnShamini Sathivel0% (1)
- MSOCHA3 Tutorial 1 Multicomponent AbsorptionDocument5 pagesMSOCHA3 Tutorial 1 Multicomponent AbsorptionTshwarelo MahlakoaneNo ratings yet
- Exp - P3 - RTD Studies in PBRDocument7 pagesExp - P3 - RTD Studies in PBRSiddesh PatilNo ratings yet
- Schx4007 Mass Transfer LabDocument60 pagesSchx4007 Mass Transfer LabAhmed AliNo ratings yet
- Lab Report Aspen Hysis UiTMDocument12 pagesLab Report Aspen Hysis UiTMAhmad SiddiqNo ratings yet
- Sequencing of Separation Columns: Direct Sequence Indirect SequenceDocument18 pagesSequencing of Separation Columns: Direct Sequence Indirect SequenceVidvendu GuptaNo ratings yet
- Mass Transfer CoefficientDocument37 pagesMass Transfer CoefficientnivedhithaNo ratings yet
- Absorption in PackedDocument21 pagesAbsorption in PackedfreakameNo ratings yet
- LleDocument30 pagesLlefirstlove_492_736373No ratings yet
- Heat Transfer Lab Manual 2015-16Document99 pagesHeat Transfer Lab Manual 2015-16Harshit Sinha100% (1)
- Liquid - Liquid Extraction in A Packed Bed: Experiment No: 2Document23 pagesLiquid - Liquid Extraction in A Packed Bed: Experiment No: 2Sameep JainNo ratings yet
- Assignment 2Document3 pagesAssignment 2deepika snehi100% (1)
- Sep Lab Exp 1 LatestDocument20 pagesSep Lab Exp 1 LatestChan Chun ChenNo ratings yet
- Cre 1 IntroductionDocument4 pagesCre 1 IntroductionEvangeline LauNo ratings yet
- Experiment: Screw Conveyor: Course Number and NameDocument10 pagesExperiment: Screw Conveyor: Course Number and NameMaheshree GohilNo ratings yet
- Mass Transfer (Topic 2)Document23 pagesMass Transfer (Topic 2)Siswand BIn Mohd Ali0% (1)
- Packed Bed AbsorptionDocument4 pagesPacked Bed AbsorptionSenthilNathanNo ratings yet
- Topic 3.2 - Internal Diffusion and ReactionDocument36 pagesTopic 3.2 - Internal Diffusion and ReactionHamdan Azman100% (1)
- Edr6 1Document10 pagesEdr6 1JudyNo ratings yet
- 04-Basics of Non-Ideal Reactors 2008Document18 pages04-Basics of Non-Ideal Reactors 2008Okky Kusumo IndradiNo ratings yet
- Chapter 1 - Part IDocument46 pagesChapter 1 - Part IMaisarah RazaliNo ratings yet
- Distillation Tutorial 1Document1 pageDistillation Tutorial 1Richardt LootsNo ratings yet
- Breakthrough CurveDocument6 pagesBreakthrough Curveema asri100% (2)
- Differential DistillationDocument8 pagesDifferential DistillationIsuru Lakmuthu MudannayakeNo ratings yet
- Isothermal Semi-Batch Reactor PPT RJC SirDocument16 pagesIsothermal Semi-Batch Reactor PPT RJC Sirsdjdsf100% (1)
- Packed Bed Distillation Column Lab ReportDocument13 pagesPacked Bed Distillation Column Lab ReportShamini Sathivel100% (6)
- Fluid Mechanics Question BankDocument6 pagesFluid Mechanics Question BankDinesh KumarNo ratings yet
- Absorption NotesDocument79 pagesAbsorption Noteshanisshi100% (2)
- UO 6 Sedimentation Study UnitDocument8 pagesUO 6 Sedimentation Study Uniteven lee100% (1)
- Vapour in Air DiffusionDocument12 pagesVapour in Air Diffusionshivangsharma105629No ratings yet
- CH 3520 Heat and Mass Transfer Laboratory: Title of The Experiment: Plate Column DistillationDocument7 pagesCH 3520 Heat and Mass Transfer Laboratory: Title of The Experiment: Plate Column DistillationVijay PrasadNo ratings yet
- Al Duri Tutorial1 AbsorptionDocument2 pagesAl Duri Tutorial1 AbsorptionJia YiNo ratings yet
- Chemical Engineering Mass Transfer NotesDocument36 pagesChemical Engineering Mass Transfer NotesLebohang Czar Nku50% (2)
- CCB 3062 Unit Operation Lab Ii Experiment 5: CSTR: TH RDDocument14 pagesCCB 3062 Unit Operation Lab Ii Experiment 5: CSTR: TH RDBhinitha ChandrasagaranNo ratings yet
- CentrifugationDocument53 pagesCentrifugationseeyo123No ratings yet
- 4.liquid2 Extraction FullDocument17 pages4.liquid2 Extraction FullMuhammad Zaidi MisniNo ratings yet
- PFR Lab ReportDocument21 pagesPFR Lab ReportValentinoDullSatin100% (1)
- Heat Transfer Equipment DesignDocument7 pagesHeat Transfer Equipment DesignBhawani Pratap Singh PanwarNo ratings yet
- 01 - PDC Study of Step Response of First Order SystemDocument8 pages01 - PDC Study of Step Response of First Order SystemNeena Regi100% (1)
- Paper Lichen Activity 3Document11 pagesPaper Lichen Activity 3Christopher MoralesNo ratings yet
- Power Plant OperationDocument0 pagesPower Plant OperationSHIVAJI CHOUDHURY100% (1)
- 6-Translocation in The PhloemDocument35 pages6-Translocation in The PhloemSultanah Khaidoo-AubdoollahNo ratings yet
- D4and5 Coulombs Law Worksheet SOLUTIONSDocument7 pagesD4and5 Coulombs Law Worksheet SOLUTIONSCss PursuerNo ratings yet
- Phthalic Anhydride Part 1Document6 pagesPhthalic Anhydride Part 1Ajay Yadav100% (1)
- Solved ISRO Scientist or Engineer Mechanical 2009 Paper With SolutionsDocument21 pagesSolved ISRO Scientist or Engineer Mechanical 2009 Paper With SolutionsAB RanaNo ratings yet
- Piping, Modules & Skids: PrefabricatedDocument2 pagesPiping, Modules & Skids: PrefabricatedRevankar B R ShetNo ratings yet
- Piu RajakDocument18 pagesPiu Rajakpiu_rajakNo ratings yet
- ST Handout 3Document23 pagesST Handout 3SwagBeast SKJJNo ratings yet
- Identifying Archaeological Metal PDFDocument4 pagesIdentifying Archaeological Metal PDFadonisghlNo ratings yet
- A Study On Some Durability Properties of Coconut Shell Aggregate ConcreteDocument13 pagesA Study On Some Durability Properties of Coconut Shell Aggregate ConcreteMa Victoria CaneteNo ratings yet
- Diagonal RelationshipDocument16 pagesDiagonal RelationshipBaiye RandolfNo ratings yet
- Ioesolutions Esign Com NP Contents Sanitary Engineering Ce 656Document5 pagesIoesolutions Esign Com NP Contents Sanitary Engineering Ce 656Ranjit MahatoNo ratings yet
- Mining: Supercritical Flow Critical Flow Subcritical FlowDocument3 pagesMining: Supercritical Flow Critical Flow Subcritical FlowBoonsita NammanaNo ratings yet
- Project Report FOR Casting Iron & Copper: PromoterDocument11 pagesProject Report FOR Casting Iron & Copper: PromoterdjchiragNo ratings yet
- Pharmacokinetics and Comparative Bioavailability of Allopurinol Formulations in Healthy SubjectsDocument5 pagesPharmacokinetics and Comparative Bioavailability of Allopurinol Formulations in Healthy SubjectsFajar NovendraNo ratings yet
- Cyclone SeparateDocument5 pagesCyclone SeparateAMARESH BADIGERNo ratings yet
- Tests On Bamboo: Compressive TestingDocument3 pagesTests On Bamboo: Compressive TestingMr.Bhaskar WabhitkarNo ratings yet
- Math Past PaperDocument10 pagesMath Past PaperARIHAN SHARMANo ratings yet
- Chap 2-2. Mixed Potential TheoryDocument16 pagesChap 2-2. Mixed Potential Theory맛있는감자No ratings yet
- Fastener Materials and NomenclatureDocument16 pagesFastener Materials and NomenclatureabhiNo ratings yet
- ME 820 - Course PlanDocument2 pagesME 820 - Course PlanArun MahalingamNo ratings yet
- Floor Cleaner Making ClassesDocument8 pagesFloor Cleaner Making ClassesMuhammad FaisalNo ratings yet
- Radiograph Interpretation - WeldsDocument6 pagesRadiograph Interpretation - WeldsPercy Morales RamirezNo ratings yet
- 2002 AriDocument53 pages2002 AriMbarouk Shaame MbaroukNo ratings yet
- Organic ChemDocument116 pagesOrganic ChemNidhi Sisodia100% (1)
- Lubricant Viscocities 140hDocument8 pagesLubricant Viscocities 140hPablo Gaspar D'Agostini AmengualNo ratings yet
Sieve Plate Distillation Column - Lab Report
Sieve Plate Distillation Column - Lab Report
Uploaded by
Shrankhla NaryaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Sieve Plate Distillation Column - Lab Report
Sieve Plate Distillation Column - Lab Report
Uploaded by
Shrankhla NaryaCopyright:
Available Formats
Experiment 1 SIEVE-PLATE DISTILLATION COLUMN
Shrankhla Narya 09001038 AIM: To find the number of theoretical stages in a Sieve Plate Distillation and calculate the overall
efficiency in a sieve plate distillation column.
APPARATUS:
Distillation Column with reboiler Automatic digital Refractometer Two fluids with different boiling point (i.e. Isopropanol and n-isopropanol) Beaker
PROCEDURE:
1. 2. 3. 4. Fill the distillation column with the two fluids- Propan-1-ol and Propan-2-ol Switch on the heater and set the temperature to around 135 C Allow the system to reach steady state Collect a few drops of distillate in a beaker and measure its refractive index using refractometer 5. Collect a few drops of residue in a beaker and measure its refractive index using refractometer 6. Repeat steps 4 and 5 every 15 minutes
THEORY:
Distillation is a method of separating the components of a solution which depends upon the distribution of the substances between a gas and a liquid phase. It is called as the workhorse of chemical industries. The basic requirement for separation is that the vapor phase composition has to be different from that of the composition of the liquid phase. Distillation mainly involves vapor liquid equilibrium. Vapors are formed in a reboiler and rise in the column. Feed is supplied in the middle, separating the column in enriching/rectifying section and stripping/exhausting section. The vapor is condensed at the top and is converted into liquid. The purpose of distillation is to get more volatile component at the top and less volatile component at the bottom. An intimate contact between the liquid and vapor phase occurs on a tray, facilitating rapid exchange of mass between them. Transport of more volatile component occurs from liquid to vapor phase while transport of less
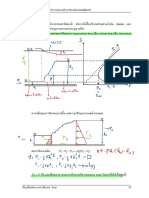
volatile component occurs from vapor to liquid phase. Thus, distillation column involves counterdiffusion of components (not necessarily equimolar). A typical distillation column looks like this:
1. Enriching Section:
Vapor rising in the section above the feed is washed with the liquid that is being recycled. The liquid coming from the condenser is leaner, less volatile as compared to the vapor rising. This causes mass transfer of less volatile component from the vapor to the liquid phase and vapor phase becomes richer in more volatile component.
2. Stripping Section:
In the section below the feed, the liquid is being stripped off of more volatile component by the vapor produced at the bottom. Liquid (coming from feed + condenser) has more volatile component and hence is stripped off by the vapor. Thus liquid now will contain less volatile component in more quantity.
3. Feed Entry:
Generally, it is the feed entry point that divides the column into rectifying section and stripping section. Feed should be introduced at a plate where operating line for enriching section cuts the operating line of the stripping section because this results in a minimum number of trays. Feed should be added at a point where its addition does not make large changes in the concentration. It should be added at a point where streams concentration matches with the feed concentration.
4. Reflux:
After the vapor from top section is condensed, some of it is sent back to the column and the rest is obtained a distillate. Reflux is the most important variable that is responsible for the high purity of the product. Without reflux, there will be no enrichment of vapor for top
section and of liquid for bottom section. More the reflux more is the purity of the distillate obtained.
5. Reflux Ratio:
Reflux ratio is the ratio of the amount of the condensed vapor which is recycled back to the column to the amount of distillate withdrawn. The reflux ratio decides how far or close the operating line is from the equilibrium curve. As the reflux ratio increases, the operating line moves away from the equilibrium curve and number of stages decreases. Total Reflux Ratio: At total reflux, minimum number of trays is required. This happens when the entire condensate is recycled back to the column the com lete li uid is re oiled no roduct withdrawn and the eed is com letel sto ed. inimum num er o sta es results when the o eratin line is at dia onal line. e lu ratio is in inite. istillate o tained is ure more volatile component and residue is pure less volatile component.
The total number of stages in case of total reflux can be calculated as under: 1. lot the e uili rium data on a s. ra h and draw a dia onal line. 2. Mark distillate and residue points on that curve. 3. Starting from distillate point, move vertically and cut the equilibrium curve, then move horizontally to cut the diagonal line. 4. Repeat step 3 until you reach the residue point. If the line at the end coincides with the residue point, then number of stages is equal to the number of steps plus one (for reboiler). 5. If line and residue point do not coincide, then does not match with the residue point, then calculate the fraction at the end. Rest is same as step 4.
OBSERVATIONS:
Temp of oil tank Temp of condensate = = C C
Distillate R.I
Residue R.I
Refractive Index of Distillate Refractive Index of Residue
= =
Experimental number of stages
CALCULATION:
Num er o sta es rom ra h Actual number of stages = 3 + 1 = 4 ( +1 for reboiler) Experimental number of stages = Overall Efficiency = =
RESULT:
Number of stages from the graph obtained are ____ and plate efficiency is ______.
CONCLUSION:
The efficiency of the column is high and is equal to 0.8. The overall efficiency of a single plate in a distilling column is defined as the ratio of the actual change in the composition of the liquid between plate n and plate n +1to the change that should occur if there were perfect equilibrium between the rising vapor and the liquid on the plate and if the rising vapor carried no entrained liquid. In general, neither of these ideal conditions is realized in practice. The typical value of plate efficiency is 0.5-0.7. So we can say that contact between liquid and vapor on the plate is good. The efficiency received is hi her in our case asicall ecause our assum tion rising vapor carried no entrained liquid did not follow.
You might also like
- Cement and Concrete Mineral Admixtures, by Tokyay M., 2016 PDFDocument325 pagesCement and Concrete Mineral Admixtures, by Tokyay M., 2016 PDFAngel Baraoed100% (3)
- Group21 Experiment11Document18 pagesGroup21 Experiment11kefiloe Letsie100% (1)
- Vapor in Air DiffusionDocument6 pagesVapor in Air DiffusionMeetNo ratings yet
- Lab6-Tubular Flow ReactorDocument11 pagesLab6-Tubular Flow ReactorNurtasha Atikah100% (1)
- CSTRDocument12 pagesCSTRsamueloNo ratings yet
- Solution:: Cosh Cosh 1 Cosh CoshDocument2 pagesSolution:: Cosh Cosh 1 Cosh CoshVaibhav GuptaNo ratings yet
- Greenwood Earnshaw Chemistry of The ElementsDocument4 pagesGreenwood Earnshaw Chemistry of The ElementsemreNo ratings yet
- Assignment (Air Pollution)Document7 pagesAssignment (Air Pollution)Durga Prasad Murmu0% (1)
- Sieve Plate Distillation ColumnDocument9 pagesSieve Plate Distillation ColumnAshish VermaNo ratings yet
- Separation Processes Lab ReportDocument15 pagesSeparation Processes Lab ReportArslanQureshi0% (1)
- Lab ManualDocument59 pagesLab ManualmarkNo ratings yet
- Exp-9 - Liquid Liquid Extraction in A Packed ColumnDocument5 pagesExp-9 - Liquid Liquid Extraction in A Packed ColumnSiddharth MohapatraNo ratings yet
- A Major Project Report On Design of Multicomponent Distillation Column by Approximate and Rigorous Method Using MatlabDocument51 pagesA Major Project Report On Design of Multicomponent Distillation Column by Approximate and Rigorous Method Using MatlabMasood HassanNo ratings yet
- CSTRDocument21 pagesCSTRirfan hilmanNo ratings yet
- Isothermal CSTR PDFDocument9 pagesIsothermal CSTR PDFprashant_cool_4_uNo ratings yet
- Sieve Plate DistillationDocument3 pagesSieve Plate DistillationRahul Pancholi67% (3)
- Theoretical Plates Calculation by McCabe-Thiele MethodDocument4 pagesTheoretical Plates Calculation by McCabe-Thiele Methodmohammad shoaibNo ratings yet
- CRE-II - Chapter-04 Fluid-Particle Systems - HKB 2.10.18Document42 pagesCRE-II - Chapter-04 Fluid-Particle Systems - HKB 2.10.18Ananya DaveNo ratings yet
- RI Vs Composition Methanol-Water MixtureDocument12 pagesRI Vs Composition Methanol-Water MixtureAnonymous VeJYFSMWLINo ratings yet
- Distillation Column DesignDocument20 pagesDistillation Column DesignSandeep Challa100% (1)
- CSTR 40LDocument16 pagesCSTR 40LhishamNo ratings yet
- Absorption and StrippingDocument60 pagesAbsorption and StrippingMyvizhi Somasundaram100% (2)
- Sieve Plate ColumnDocument14 pagesSieve Plate ColumnShamini Sathivel0% (1)
- MSOCHA3 Tutorial 1 Multicomponent AbsorptionDocument5 pagesMSOCHA3 Tutorial 1 Multicomponent AbsorptionTshwarelo MahlakoaneNo ratings yet
- Exp - P3 - RTD Studies in PBRDocument7 pagesExp - P3 - RTD Studies in PBRSiddesh PatilNo ratings yet
- Schx4007 Mass Transfer LabDocument60 pagesSchx4007 Mass Transfer LabAhmed AliNo ratings yet
- Lab Report Aspen Hysis UiTMDocument12 pagesLab Report Aspen Hysis UiTMAhmad SiddiqNo ratings yet
- Sequencing of Separation Columns: Direct Sequence Indirect SequenceDocument18 pagesSequencing of Separation Columns: Direct Sequence Indirect SequenceVidvendu GuptaNo ratings yet
- Mass Transfer CoefficientDocument37 pagesMass Transfer CoefficientnivedhithaNo ratings yet
- Absorption in PackedDocument21 pagesAbsorption in PackedfreakameNo ratings yet
- LleDocument30 pagesLlefirstlove_492_736373No ratings yet
- Heat Transfer Lab Manual 2015-16Document99 pagesHeat Transfer Lab Manual 2015-16Harshit Sinha100% (1)
- Liquid - Liquid Extraction in A Packed Bed: Experiment No: 2Document23 pagesLiquid - Liquid Extraction in A Packed Bed: Experiment No: 2Sameep JainNo ratings yet
- Assignment 2Document3 pagesAssignment 2deepika snehi100% (1)
- Sep Lab Exp 1 LatestDocument20 pagesSep Lab Exp 1 LatestChan Chun ChenNo ratings yet
- Cre 1 IntroductionDocument4 pagesCre 1 IntroductionEvangeline LauNo ratings yet
- Experiment: Screw Conveyor: Course Number and NameDocument10 pagesExperiment: Screw Conveyor: Course Number and NameMaheshree GohilNo ratings yet
- Mass Transfer (Topic 2)Document23 pagesMass Transfer (Topic 2)Siswand BIn Mohd Ali0% (1)
- Packed Bed AbsorptionDocument4 pagesPacked Bed AbsorptionSenthilNathanNo ratings yet
- Topic 3.2 - Internal Diffusion and ReactionDocument36 pagesTopic 3.2 - Internal Diffusion and ReactionHamdan Azman100% (1)
- Edr6 1Document10 pagesEdr6 1JudyNo ratings yet
- 04-Basics of Non-Ideal Reactors 2008Document18 pages04-Basics of Non-Ideal Reactors 2008Okky Kusumo IndradiNo ratings yet
- Chapter 1 - Part IDocument46 pagesChapter 1 - Part IMaisarah RazaliNo ratings yet
- Distillation Tutorial 1Document1 pageDistillation Tutorial 1Richardt LootsNo ratings yet
- Breakthrough CurveDocument6 pagesBreakthrough Curveema asri100% (2)
- Differential DistillationDocument8 pagesDifferential DistillationIsuru Lakmuthu MudannayakeNo ratings yet
- Isothermal Semi-Batch Reactor PPT RJC SirDocument16 pagesIsothermal Semi-Batch Reactor PPT RJC Sirsdjdsf100% (1)
- Packed Bed Distillation Column Lab ReportDocument13 pagesPacked Bed Distillation Column Lab ReportShamini Sathivel100% (6)
- Fluid Mechanics Question BankDocument6 pagesFluid Mechanics Question BankDinesh KumarNo ratings yet
- Absorption NotesDocument79 pagesAbsorption Noteshanisshi100% (2)
- UO 6 Sedimentation Study UnitDocument8 pagesUO 6 Sedimentation Study Uniteven lee100% (1)
- Vapour in Air DiffusionDocument12 pagesVapour in Air Diffusionshivangsharma105629No ratings yet
- CH 3520 Heat and Mass Transfer Laboratory: Title of The Experiment: Plate Column DistillationDocument7 pagesCH 3520 Heat and Mass Transfer Laboratory: Title of The Experiment: Plate Column DistillationVijay PrasadNo ratings yet
- Al Duri Tutorial1 AbsorptionDocument2 pagesAl Duri Tutorial1 AbsorptionJia YiNo ratings yet
- Chemical Engineering Mass Transfer NotesDocument36 pagesChemical Engineering Mass Transfer NotesLebohang Czar Nku50% (2)
- CCB 3062 Unit Operation Lab Ii Experiment 5: CSTR: TH RDDocument14 pagesCCB 3062 Unit Operation Lab Ii Experiment 5: CSTR: TH RDBhinitha ChandrasagaranNo ratings yet
- CentrifugationDocument53 pagesCentrifugationseeyo123No ratings yet
- 4.liquid2 Extraction FullDocument17 pages4.liquid2 Extraction FullMuhammad Zaidi MisniNo ratings yet
- PFR Lab ReportDocument21 pagesPFR Lab ReportValentinoDullSatin100% (1)
- Heat Transfer Equipment DesignDocument7 pagesHeat Transfer Equipment DesignBhawani Pratap Singh PanwarNo ratings yet
- 01 - PDC Study of Step Response of First Order SystemDocument8 pages01 - PDC Study of Step Response of First Order SystemNeena Regi100% (1)
- Paper Lichen Activity 3Document11 pagesPaper Lichen Activity 3Christopher MoralesNo ratings yet
- Power Plant OperationDocument0 pagesPower Plant OperationSHIVAJI CHOUDHURY100% (1)
- 6-Translocation in The PhloemDocument35 pages6-Translocation in The PhloemSultanah Khaidoo-AubdoollahNo ratings yet
- D4and5 Coulombs Law Worksheet SOLUTIONSDocument7 pagesD4and5 Coulombs Law Worksheet SOLUTIONSCss PursuerNo ratings yet
- Phthalic Anhydride Part 1Document6 pagesPhthalic Anhydride Part 1Ajay Yadav100% (1)
- Solved ISRO Scientist or Engineer Mechanical 2009 Paper With SolutionsDocument21 pagesSolved ISRO Scientist or Engineer Mechanical 2009 Paper With SolutionsAB RanaNo ratings yet
- Piping, Modules & Skids: PrefabricatedDocument2 pagesPiping, Modules & Skids: PrefabricatedRevankar B R ShetNo ratings yet
- Piu RajakDocument18 pagesPiu Rajakpiu_rajakNo ratings yet
- ST Handout 3Document23 pagesST Handout 3SwagBeast SKJJNo ratings yet
- Identifying Archaeological Metal PDFDocument4 pagesIdentifying Archaeological Metal PDFadonisghlNo ratings yet
- A Study On Some Durability Properties of Coconut Shell Aggregate ConcreteDocument13 pagesA Study On Some Durability Properties of Coconut Shell Aggregate ConcreteMa Victoria CaneteNo ratings yet
- Diagonal RelationshipDocument16 pagesDiagonal RelationshipBaiye RandolfNo ratings yet
- Ioesolutions Esign Com NP Contents Sanitary Engineering Ce 656Document5 pagesIoesolutions Esign Com NP Contents Sanitary Engineering Ce 656Ranjit MahatoNo ratings yet
- Mining: Supercritical Flow Critical Flow Subcritical FlowDocument3 pagesMining: Supercritical Flow Critical Flow Subcritical FlowBoonsita NammanaNo ratings yet
- Project Report FOR Casting Iron & Copper: PromoterDocument11 pagesProject Report FOR Casting Iron & Copper: PromoterdjchiragNo ratings yet
- Pharmacokinetics and Comparative Bioavailability of Allopurinol Formulations in Healthy SubjectsDocument5 pagesPharmacokinetics and Comparative Bioavailability of Allopurinol Formulations in Healthy SubjectsFajar NovendraNo ratings yet
- Cyclone SeparateDocument5 pagesCyclone SeparateAMARESH BADIGERNo ratings yet
- Tests On Bamboo: Compressive TestingDocument3 pagesTests On Bamboo: Compressive TestingMr.Bhaskar WabhitkarNo ratings yet
- Math Past PaperDocument10 pagesMath Past PaperARIHAN SHARMANo ratings yet
- Chap 2-2. Mixed Potential TheoryDocument16 pagesChap 2-2. Mixed Potential Theory맛있는감자No ratings yet
- Fastener Materials and NomenclatureDocument16 pagesFastener Materials and NomenclatureabhiNo ratings yet
- ME 820 - Course PlanDocument2 pagesME 820 - Course PlanArun MahalingamNo ratings yet
- Floor Cleaner Making ClassesDocument8 pagesFloor Cleaner Making ClassesMuhammad FaisalNo ratings yet
- Radiograph Interpretation - WeldsDocument6 pagesRadiograph Interpretation - WeldsPercy Morales RamirezNo ratings yet
- 2002 AriDocument53 pages2002 AriMbarouk Shaame MbaroukNo ratings yet
- Organic ChemDocument116 pagesOrganic ChemNidhi Sisodia100% (1)
- Lubricant Viscocities 140hDocument8 pagesLubricant Viscocities 140hPablo Gaspar D'Agostini AmengualNo ratings yet