Professional Documents
Culture Documents
Bài 5
Bài 5
Uploaded by
Tuan TranCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Bài 5
Bài 5
Uploaded by
Tuan TranCopyright:
Available Formats
Bi 5: THN KINH
A. Phn tch cung phn x:
1. ngha, mc ch:
Phn x l phn ng ca c th i vi mi dng kch thch t mi trng. l nguyn tc hot ng bao trm v xuyn sut i sng mi c th. Mi phn x u phi c mt cung phn x tng ng. Cung phn x bao gm 5 yu t l : Th quan Dy thn kinh hng tm Trung ng thn kinh Dy thn kinh ly tm C quan thc hin (hay tc quan) V sau ngi ta cn cng nhn thm mt yu t th 6 trong cung phn x l ng hng tm ngc t tc quan v trung ng. Phn x ch thc hin c khi cc yu t ca cung phn x nguyn vn c v cu to v chc nng. Thi gian t khi kch thch tc ng n khi phn x xy ra c gi l thi gian phn x hay thi gian tim tng. Trn mt s ch phm ch-ty , c th o tnh c thi gian cng nh phn tch cc yu t thnh phn ca cung phn x.
2. Kt qu, gii thch:
ch khng chc ty, dng khn qun ch v dng ko ln ct ngang u ch pha di 2 mt. Mc hm di v treo ln gi th nghim. Thm mu vt ct, ch yn tnh mt thi gian, hai chn thng xung Trng hp ch cha b lt da chn v b lt da chn:
Thi gian phn x (s) Nng ha cht H2SO4 0.5% H2SO4 1% ch cha lt da chn Ln 1 2s40 9s00 Ln 2 2s75 8s62 Ln 3 2s50 8s70 Trung bnh ch lt da chn Ln Ln Ln Trung 1 2 3 bnh 2s00 1s54 1s50 10s31 10s46 18s83
Gii thch: Trn l thuyt thi gian phn x co chn ca ch nng axit cao (H2SO4 1%) s din ra nhanh hn nng axit thp (H2SO4 0,5%) nhng khi tin hnh th nghim ta khng thu c kt qu nh th v: Trong th nghim ny ch s dng mt con ch th nghim nn chnh xc khng cao
Do ch b lt da chn nn cc t bo th cm trn da cng b mt v th cho nn khi nhng chn ch vo trong dung dch H2SO4 th ta thy thi gian phn x ca ch chm hn khi cha lt da Trng hp tc ng ln dy thn kinh hng c i:
Nng ha cht H2SO4 0.5% H2SO4 1%
Thi gian phn x (s) Bnh thng 1s 0s43 Tht ch dy thn kinh hng Khng phn x Khng phn x Ct ch dy thn kinh hng Khng phn x Khng phn x
Gii thch: Khi tch dy thn kinh, thm t dung dch sinh l th cu to v chc nng ca dy thn kinh vn gi nguyn, do phn x vn xy ra Sau dng ch lun di v tht cht (1 nt) dy thn kinh hng. ch khng phn x do kch thch truyn qua dy thn kinh b nghn li. Sau khi ct ch, tm dung dch sinh l cho dy thn kinh hng c hai i. ch khng phn x do kch thch truyn qua dy thn kinh vn b nghn li. Do thi gian th nghim l c hn nn c th thi gian phn x s lu, nu c th do ch phm thn kinh c kh nng hng phn. Ngha l c cm ng ch khng phi l phn x Trng hp dng ether v tinh th mui n tc dng ln dy thn kinh hng i: (dng dung dch acid H2SO4 0.5%)
Thi gian phn x (s) Chn th nht Trc khi tm Sau khi tm ether ether 2s05s 20s30
Gii thch: Chn th nht: Trc khi tm ether ch vn phn x co chn vi acid bnh thng Sau khi tm ether 5 pht, thi gian phn x ca ch lu hn. Do ether l cht gy m nn khi cho ether tc ng ln dy thn kinh hng th n s lm c ch dy thn kinh dn n ch c phn x vi acid chm Chn th hai: Trc khi tm mui n ch vn phn x co chn vi acid bnh thng nh trn Sau khi tm mui n 5 pht, thi gian phn x ca ch lu hn. Do mui n ( NaCl) khi tm vo dy thn kinh ch c tc dng c ch lm chm s lan truyn xung thn kinh ti trung ng thn kinh v ty sng dn ti ch phn x chm so vi trc khi t tinh th mui n ln dy thn kinh hng i. V: NaCl s phn li thnh Na+ v
Chn th hai Trc khi tm Sau khi tm tinh tinh th mui th mui 2s15 21s05
Cl- bn ngoi mng t bo, cn bn trong t bo c: K+ , Cl- . Khi kch thch ch th knh Natri s m, thng thng Natri bn ngoi mng t bo s i vo trong t bo do chnh lch in th hot ng do Cl- bn trong t bo ht vo, nhng lc by bn ngoi t bo cng xut hin Cl- v th Na+ di chuyn vo bn trong t bo s chm. Lm chm s lan truyn xung thn kinh nn ch phn x chm hn. Trng hp ct u ch: (dng dung dch acid H2SO4 0.5%)
Thi gian phn x (s) Chn th nht Tch dy tk hng 8s35
Ct dy tk hng Khng phn x
Chn th hai Trc khi chc Sau khi chc ty ty 4s00 Khng phn x
Gii thch: Chn th nht: M v tch dy thn kinh hng: Qua th nghim trn ta thy ch vn c phn x co chn vi acid tuy nhin phn x ny phi cn nhiu thi gian Ct dy thn kinh hng: Khi b ct dy thn kinh th ch khng phn x so vi khi cha ct dy thn kinh. Do khi ct dy thn kinh hng th s lm ct t s truyn cc kch thch t cc t bo th quan n trung ng thn kinh v ty sng. Chn th hai: Gi nguyn vn (trc khi chc ty) : th phn x co chn vi acid Sau dng di chc ty chc thng vo ty sng ti vt ct ngang u th khi th li phn x co chn vi acid th ch khng cn phn x co chn. Do khi chc thng vo ty sng ph hy hon ton c quan no b cn li sau khi ct u. Lc ny cc yu t ca cung phn x khng cn nguyn vn nn phn x khng thc hin c.
B.Quan st cc phn x trng lc. Phn x ca ty sng:
1. ngha, mc ch:
Cc phn x trng lc l nhng phn x co c ko di gi thng bng v duy tr t th ca c th trong khng gian. Phn x trng lc c iu khin t nhiu cu trc khc nhau ca h thn kinh trung ng t ty sng tr ln. Khi ct t s lin h t ty sng n cu trc khc ca no b,cc phn x trng lc s tng cng. ng vt thang tin ha thp, c tnh c lp ca ty sng trong cc phn x trng lc v mt s phn x n gin nh phn x gn, phn x da biu hin r rng hn so vi ng vt thang tin ha cao. To mt ch phm ch ty c th quan st c cc phn x trng lc v phn x da ch. Cn quan st cc phn x trng lc phn x ca ty sng khi ct t s lin h ca ty sng vi cc cu trc khc ca no b.
2. Kt qu, gii thch:
Mc hm di ch v treo ln th nghim. Ch mt thi gian cho ch yn tnh ri quan st: hai chn sau ca ch dui thng Dng ko ct ngang hm trn ca ch t pha di hai mt tr ln. Thm mu, ch cho ch yn tnh ri quan st: hai chn ch khng dui thng na m hi co ln. Gii thch: Con ch b cht mt u vn co dt chn li. Trong trng hp ny phn thn ca con vt phn ng mt cch my mc, cn phn no b hy b. chnh l phn x trng lc c do ty sng iu khin khi ct b phn no pha trn. Tho ch khi mc v t ch t th ngi khoanh 2 chn sau trc bng trn bn m. Bnh thng khi cha ct hm trn (cn nguyn no), ch khng th ngi t th ny. Gii thch: chnh l phn x trng lc ca c do ty sng iu khin, cn gi l phn x bt t th ngi ca ch, dng panh gp ming bng thm tm acid H2SO4 0.5% v t ln vng da bng ta thy ch dng chi trc gt nh ming bng cng bn. Khi gp ming bng thm tm acid H2SO4 1%, ch dng c hai chi trc gt ming bng, sau dy mnh v ng xung Gii thch: 2 phn x trn l phn x da (cn gi l phn x gi) do ty sng iu khin. Ty iu kin kch thch, s lng cc on ty sng than gia phn x s thay i, cng kch thch cng mnh, cng c nhiu on ty sng tham gia.
C. c ch Sechenov:
1. ngha, mc ch:
M ch quan st i no ca ch. Tm hiu cc tc dng thm thu ca mui n i no, mt qu trnh c ch xut hin i vi nhiu cu trc khc nh ty sng, lm chm cc phn x co c.
2. Kt qu, gii thch:
Sau khi t tinh th NaCl vo i no ch. Dng dung dch H2SO4 1% o thi gian phn x co chn ch
Khng c mui n 2s30
Thi gian phn x (s) C mui n 4s50
Ra sch mui n 3s50
So snh : Da vo bng s liu trn ta nhn thy :
Sau khi t tinh th NaCl vo i no th ch phn x vi acid H2SO4 1% chm hn so vi trc khi cho mui n vo Khi ra sch mui n v th phn x li vi acid H2SO4 1% th thi gian phn x cng chm hn so vi trc khi cho mui n vo, nhng vn nhanh hn so vi lc c mui n Nh vy, trong 3 trng hp th trng hp c s tc ng ca mui n ln i no ch lm cho phn x co chn ch din ra lu hn 2 trng hp cn li. Gii thch: Mng sinh cht ca cc t bo thn kinh c cha bm Na+ K+ ATPase rt hot ng lm cho Na+ ra ngoi v K+ vo trong,iu ny to nn mt gradient nng i vi Na+,K+ v Cl-. Nh ta bit, Na+v Cl- u c xu hng tch t bn ngoi t bo.Khi ta t tinh th mui n (NaCl) vo i no ca ch lm cho nng Na+ v Cl- tng ln lm thay i gradient nng ca t bo thn kinh,khi s chnh lch v nng gia cc ion l qua ln s dn n vic dn truyn cc xung thn kinh yu i dn n vic phn x vi acid ca chn ch chm i. l l do v sao khi ta t mui n vo i no th phn x ca ch chm i. Khi ra sch mui n th nng Na+ v Cl- gim bt nn thi gian phn x nhanh hn so vi lc c mui n
D. Th nghim ph tiu no :
1. Mc ch:
Nm r chc nng ca tiu no M, quan st ri ct b tng phn v ton b tiu no thy s ri lon vn ng ca ch
2. Kt qu, gii thch:
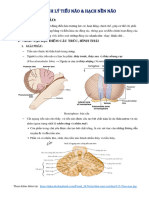
Sau khi quan st cc cu trc khc nhau ca no ch. Dng panh nh gp nh nhng ph hy tiu no phi ca ch. Ta thy ch nghing u v pha tiu no b ct (bn phi). Khi th vo thau nc th ch bi vng trn v pha b ct (bn phi) Dng panh gp tip na tiu no pha bn tri, ta thy ch chi u xung t v khng bi, khng nhy Gii thch:Do tiu no c chc nng quan trng trong vic iu ha cc phn x trng lc c nhm gi t th v thng bng cho c th. Mt khc tiu no iu ha cc phn x co c theo mun, phi hp cc ng tc cho ng tm, ng hng, lm cho s vn ng chnh xc hn. Khi ta ct b mt phn tiu no ngay lp tc s vn ng ca ch b ri lon, bn pha b ct tiu no th cc chi bn khng hot ng, cn cc chi bn pha cn li th hot ng bnh thng nn ch ch bi v pha bn tiu no b ct.
You might also like
- 1.Phân Tích Cung Phản Xạ TủyDocument19 pages1.Phân Tích Cung Phản Xạ TủyPhú MinhhNo ratings yet
- CẢM ỨNG Ở ĐỘNG VẬTDocument3 pagesCẢM ỨNG Ở ĐỘNG VẬTduc ducNo ratings yet
- Sinh CK2 TsDocument7 pagesSinh CK2 TsJane HuynhNo ratings yet
- N T P Ki M Tra 11a1Document4 pagesN T P Ki M Tra 11a1Thùy PhạmNo ratings yet
- SINH LÍ HỌC THẦN KINHDocument21 pagesSINH LÍ HỌC THẦN KINHChâu MinhNo ratings yet
- Báo Cáo TH 8 9Document9 pagesBáo Cáo TH 8 9Mai PhạmNo ratings yet
- Đề cương giải phẫu kì 1 năm 2Document3 pagesĐề cương giải phẫu kì 1 năm 2Hiền AnhNo ratings yet
- SINH LÝ HỆ THẦN KINH VẬN ĐỘNGDocument18 pagesSINH LÝ HỆ THẦN KINH VẬN ĐỘNGDuc NguyenNo ratings yet
- FILE - 20220110 - 184016 - de Cuong Cuoi hk1Document2 pagesFILE - 20220110 - 184016 - de Cuong Cuoi hk1ngocvuthanh1290No ratings yet
- c2 Thêm Sinh Ly Than Kinh Cao Cap Updatẹ.5.2020Document10 pagesc2 Thêm Sinh Ly Than Kinh Cao Cap Updatẹ.5.2020Mỹ Duyên Nguyễn ThịNo ratings yet
- Nút Bu C StaniusDocument5 pagesNút Bu C StaniusYến VũNo ratings yet
- ôn tập SLHĐTKDocument16 pagesôn tập SLHĐTKphamhuyentrang1032003No ratings yet
- ĐỀ 1Document9 pagesĐỀ 1Linhh ĐâyyNo ratings yet
- PHẦN II - PHẢN XẠ CÓ ĐIỀU KIỆN (BÀI 6)Document7 pagesPHẦN II - PHẢN XẠ CÓ ĐIỀU KIỆN (BÀI 6)anhtu11104No ratings yet
- Sinh Lý Tre emDocument7 pagesSinh Lý Tre empet.ha HoangNo ratings yet
- Ôn tậpDocument40 pagesÔn tậpNgọc PhạmNo ratings yet
- De Thi HSG Lop 8 Co Dap AnDocument89 pagesDe Thi HSG Lop 8 Co Dap AnKHANHPHUONG PHẠMNo ratings yet
- Copy of Sinh Lí Động Vật-2014Document29 pagesCopy of Sinh Lí Động Vật-2014Lương NhưNo ratings yet
- Câu Hỏi Tham Khảo Slđv 2014Document28 pagesCâu Hỏi Tham Khảo Slđv 201428. Phan Hải ĐăngNo ratings yet
- SINH LÝ HỆ THẦN KINH CẢM GIÁCDocument30 pagesSINH LÝ HỆ THẦN KINH CẢM GIÁCDuc NguyenNo ratings yet
- CƠ 2 - Điều hoà lực cơ & Chuyển hoá ở cơ vânDocument7 pagesCƠ 2 - Điều hoà lực cơ & Chuyển hoá ở cơ vânMy VõNo ratings yet
- Sinh Ly Cac Giac QuanDocument7 pagesSinh Ly Cac Giac QuanPhạm Xuân Phúc NguyênNo ratings yet
- TRƯỜNG THPT CHUYÊN LÊ KHIẾTDocument19 pagesTRƯỜNG THPT CHUYÊN LÊ KHIẾTimnotoverthinking28012008No ratings yet
- Ôn Tập Thi 45' - Sinh 11 HKII.2022Document10 pagesÔn Tập Thi 45' - Sinh 11 HKII.2022ReC - Reporter ClubNo ratings yet
- Ôn tập sinh lý thần kinhDocument30 pagesÔn tập sinh lý thần kinhbuicongkhoauwuNo ratings yet
- 10 - NGÂN HÀNG 11 - - 2.0 - - 1780 - - BÀI - - - CẢM ỨNG ĐV - TẬP TÍNH - - 1 CỘTDocument49 pages10 - NGÂN HÀNG 11 - - 2.0 - - 1780 - - BÀI - - - CẢM ỨNG ĐV - TẬP TÍNH - - 1 CỘTNguyễn KhôiNo ratings yet
- Bản Sao Của CHUONG SL CƠDocument10 pagesBản Sao Của CHUONG SL CƠnguyenhuy2004dnNo ratings yet
- Tên đề tài: "Bài Thu Hoạch Thực Hành Sinh Lý Gia Súc"Document12 pagesTên đề tài: "Bài Thu Hoạch Thực Hành Sinh Lý Gia Súc"T. Thắm100% (1)
- 30 de Thi HSG Cap HuyenDocument100 pages30 de Thi HSG Cap HuyenNguyễn Thanh HuyềnNo ratings yet
- Phản xạ phế vịDocument7 pagesPhản xạ phế vịGdfgdFdfdfNo ratings yet
- 1. Đề Thi HSG Sinh 8Document29 pages1. Đề Thi HSG Sinh 8Linhh ĐâyyNo ratings yet
- 2-CẢM ỨNG Ở ĐỘNG VẬTDocument2 pages2-CẢM ỨNG Ở ĐỘNG VẬTBảoHânNo ratings yet
- BUỔI THỨ NHẤTDocument20 pagesBUỔI THỨ NHẤTKhoa PhạmNo ratings yet
- Ôn tập hsg sinh 8Document5 pagesÔn tập hsg sinh 8huong nguyenNo ratings yet
- LEC4 CHỨC NĂNG VẬN ĐỘNG TỦY SỐNGDocument6 pagesLEC4 CHỨC NĂNG VẬN ĐỘNG TỦY SỐNGVân Đào HồngNo ratings yet
- Bài tổng hợp môn GIẢI PHẪUDocument10 pagesBài tổng hợp môn GIẢI PHẪUHiền AnhNo ratings yet
- Sinh 11 de Cuong Cuoi Ki 2Document10 pagesSinh 11 de Cuong Cuoi Ki 2Thùyy LinhhNo ratings yet
- Bài 1Document26 pagesBài 1nghia5802 -CBRO sv1No ratings yet
- Tailieuxanh Tiet 24 2126Document4 pagesTailieuxanh Tiet 24 2126Quangg VũNo ratings yet
- Ôn tập sinh 8Document16 pagesÔn tập sinh 8dq leNo ratings yet
- KHTN 8 - de Cuong KtgkiiDocument6 pagesKHTN 8 - de Cuong KtgkiiMINH TRAN THI NGOCNo ratings yet
- Sinh Lý GuytonDocument3 pagesSinh Lý GuytonCon BầuNo ratings yet
- (123doc) - Phan-Xa-Sinh-Ly-Than-Kinh-Cap-CaoDocument49 pages(123doc) - Phan-Xa-Sinh-Ly-Than-Kinh-Cap-CaoDam Ngoc AnhNo ratings yet
- I. Chủ nghĩa hành vi và điều hòa cổ điểnDocument10 pagesI. Chủ nghĩa hành vi và điều hòa cổ điểnPhươngNo ratings yet
- Bài 5. Hệ Thần Kinh Cao Cấp 2024Document22 pagesBài 5. Hệ Thần Kinh Cao Cấp 2024Nguyễn Đức HuyNo ratings yet
- MỘT SỐ CHỨC NĂNG CẤP CAO CỦA HỆ THẦN KINHDocument15 pagesMỘT SỐ CHỨC NĂNG CẤP CAO CỦA HỆ THẦN KINHLê TúNo ratings yet
- ÔN TẬP SINH HỌC GIỮA KÌ I1Document5 pagesÔN TẬP SINH HỌC GIỮA KÌ I1ntn33207No ratings yet
- On Thi Tot Nghiep THPT Mon Sinh Hoc Chuyen de Cam Ung o Dong VatDocument10 pagesOn Thi Tot Nghiep THPT Mon Sinh Hoc Chuyen de Cam Ung o Dong VatBảo ChangNo ratings yet
- SINH LÝ TIỂU NÃODocument14 pagesSINH LÝ TIỂU NÃOnguyentrongan.dngNo ratings yet
- CHUẨN SINH HỌC 11 HỌC KỲ 2Document10 pagesCHUẨN SINH HỌC 11 HỌC KỲ 2Phạm Thùy LinhNo ratings yet
- Câu Hỏi Trắc NghiệmDocument8 pagesCâu Hỏi Trắc NghiệmDo Thi Tuong VyNo ratings yet
- Ôn Tập Ghkii Sinh11 (2023-2024)Document11 pagesÔn Tập Ghkii Sinh11 (2023-2024)Hạnh PhanNo ratings yet
- De Thi HSG Lop 11 2017 2018 Duyen Hai Sinh HocDocument4 pagesDe Thi HSG Lop 11 2017 2018 Duyen Hai Sinh HocNguyễn ThuNo ratings yet
- GHP1 Đã SoátDocument11 pagesGHP1 Đã SoátDuc AnhNo ratings yet
- Câu Hỏi Tham Khảo Giữa Kì II Môn Sinh 11Document5 pagesCâu Hỏi Tham Khảo Giữa Kì II Môn Sinh 11ngandinh1701No ratings yet
- Sinh Lý Thân NãoDocument9 pagesSinh Lý Thân Nãonguyentrongan.dngNo ratings yet
- BÀI 17. CẢM ỨNG Ở ĐỘNG VẬTDocument26 pagesBÀI 17. CẢM ỨNG Ở ĐỘNG VẬTGia Huy TrầnNo ratings yet
- TỰ LUẬN PHẦN CẢM ỨNG Ở ĐỘNG VẬTDocument2 pagesTỰ LUẬN PHẦN CẢM ỨNG Ở ĐỘNG VẬTNhân Châu Nguyễn HồNo ratings yet