Professional Documents
Culture Documents
Assignment 2 Follow Up 3
Assignment 2 Follow Up 3
Uploaded by
gillnumber220 ratings0% found this document useful (0 votes)
22 views1 pageThe document presents equations for calculating the equilibrium constant K for a reaction involving NO2 and N2O4. It gives values for the partial pressures and concentrations of the reactants and products at equilibrium. It then shows the calculation of solving the equation K/Ptot*(0.4 + ξ)*(0.2 - ξ) = (0.2 + 2ξ)2 for the value of ξ, using K = 0.296.
Original Description:
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document presents equations for calculating the equilibrium constant K for a reaction involving NO2 and N2O4. It gives values for the partial pressures and concentrations of the reactants and products at equilibrium. It then shows the calculation of solving the equation K/Ptot*(0.4 + ξ)*(0.2 - ξ) = (0.2 + 2ξ)2 for the value of ξ, using K = 0.296.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
22 views1 pageAssignment 2 Follow Up 3
Assignment 2 Follow Up 3
Uploaded by
gillnumber22The document presents equations for calculating the equilibrium constant K for a reaction involving NO2 and N2O4. It gives values for the partial pressures and concentrations of the reactants and products at equilibrium. It then shows the calculation of solving the equation K/Ptot*(0.4 + ξ)*(0.2 - ξ) = (0.2 + 2ξ)2 for the value of ξ, using K = 0.296.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 1
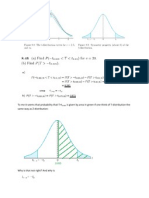
K = ((PNO2)/Po)2/(PN2O4/Po) = (PNO2)2/PN2O4 *1/Po
K = ((0,2 + 2)/(0,4 + )*Ptot)2/((0,2 )/(0,4 + )*Ptot) *1/Po
K = (0,2 + 2)2/((0,4 + )*(0,2 )) *Ptot/(Po), Po = 1 bar
K/Ptot* (0,4 + )*(0,2 ) = (0,2 + 2)2 , K = 0,296 (fra opg. 1)
20/20*(0,08 -0,2 2) = 0,04 + 0,8 + 42
5 2 + 0,04 = 0 => = 0.034164
During your first answer you said that there was an error for K in this orange part. I tried to change K
to K = 0,296 but I did not get an right answer for . Can you show calculations from here:
K/Ptot* (0,4 + )*(0,2 ) = (0,2 + 2)2 , K = 0,296 (fra opg. 1)
0.296/20*(0,08 -0,2 2) = 0,04 + 0,8 + 42
And so on?
You might also like
- Solutions To Problems, Capitulo 2 LevenspielDocument6 pagesSolutions To Problems, Capitulo 2 LevenspielAlexander Gonzalez Romero75% (4)
- Corrosion and Surface Chemistry of Metals: Solution0DQXDODocument51 pagesCorrosion and Surface Chemistry of Metals: Solution0DQXDOFrederick EstabilloNo ratings yet
- While: %2 Filas 4 ColumnasDocument2 pagesWhile: %2 Filas 4 ColumnasSergio LunaNo ratings yet
- JP XII Physical&Inorganic Chemistry (16) - Prev Chaps + Inorg. Chem PDFDocument14 pagesJP XII Physical&Inorganic Chemistry (16) - Prev Chaps + Inorg. Chem PDFSudhanshu BharadwajNo ratings yet
- JEE Main 2019 Chemistry January Attempt Shift - 1 (11th January, 2019)Document16 pagesJEE Main 2019 Chemistry January Attempt Shift - 1 (11th January, 2019)Resonance Eduventures100% (3)
- Adva PI SolDocument11 pagesAdva PI SolharshNo ratings yet
- 09-JEE-Adv Grand Test 09 Solutions (P 2)Document13 pages09-JEE-Adv Grand Test 09 Solutions (P 2)Ranjan PrasadNo ratings yet
- Equilibrium StateDocument2 pagesEquilibrium StateTechie RTNo ratings yet
- ChemistryDocument15 pagesChemistryGautam VermaNo ratings yet
- Pre-Lecture Announcements: CN5010 Mathematical Methods in Chemical EngineeringDocument28 pagesPre-Lecture Announcements: CN5010 Mathematical Methods in Chemical EngineeringAnonymous kejOID9QsNo ratings yet
- Ch17 Ch20 SolutionsDocument25 pagesCh17 Ch20 SolutionsmamaemtolokoNo ratings yet
- Equilibrium WsDocument2 pagesEquilibrium WsBianca RolstonNo ratings yet
- Redox Reactions Mini QuestionsDocument5 pagesRedox Reactions Mini QuestionsKL KNo ratings yet
- TARGET: JEE (Main) 2017: NO. 20 Course: ABHINAV (EA)Document4 pagesTARGET: JEE (Main) 2017: NO. 20 Course: ABHINAV (EA)Jyöt Sîlvēr67% (3)
- Photocatalysts Chart DiRoccoDocument1 pagePhotocatalysts Chart DiRoccoDevin FergusonNo ratings yet
- Adobe Scan 09-Dec-2023Document7 pagesAdobe Scan 09-Dec-2023das403717No ratings yet
- Dep Questions: Acel Dbe Dollb WingDocument6 pagesDep Questions: Acel Dbe Dollb WingCHETAN SETIYANo ratings yet
- UW Equilibrium WS Key PDFDocument7 pagesUW Equilibrium WS Key PDFKiki ShofiaNo ratings yet
- Algo Assignment 3 SolutionDocument6 pagesAlgo Assignment 3 SolutionTasfiq JahidNo ratings yet
- Bakliwal Tutorials - IIT: ChemistryDocument1 pageBakliwal Tutorials - IIT: ChemistrySACHIN KASERANo ratings yet
- CHEMISTRY Revision DPP 3 SolutionDocument4 pagesCHEMISTRY Revision DPP 3 SolutionPraphul Pulkit GiriNo ratings yet
- Omborder-91 of 2022270120230911Document12 pagesOmborder-91 of 2022270120230911kannanchammyNo ratings yet
- Homework 2Document11 pagesHomework 2Roberta ResendeNo ratings yet
- 153713-08 Jan Shift 02-Chemistry-V1Document9 pages153713-08 Jan Shift 02-Chemistry-V1hithereiammisusingwhatsappNo ratings yet
- Electronic Configuration: GPTC Kothamangalam Selvarajan T RDocument5 pagesElectronic Configuration: GPTC Kothamangalam Selvarajan T Rweak manNo ratings yet
- sm5 139Document1 pagesm5 139Sadie HnatowNo ratings yet
- Major Test-6 PDFDocument34 pagesMajor Test-6 PDFrtaweryeryNo ratings yet
- Ass 4Document4 pagesAss 4Duy Do MinhNo ratings yet
- Ass 4Document4 pagesAss 4Duy Do MinhNo ratings yet
- CBO-4 ResoSir Qt0q96aDocument33 pagesCBO-4 ResoSir Qt0q96aL GoldenmasterNo ratings yet
- B.Mat Part Test 3: IIT 2011 Pt3/Cmp/P (Ii) /solnsDocument26 pagesB.Mat Part Test 3: IIT 2011 Pt3/Cmp/P (Ii) /solnsSarvesh DubeyNo ratings yet
- Dpaj Aeeede: D - O) DH-G)Document10 pagesDpaj Aeeede: D - O) DH-G)Simone bbbbeNo ratings yet
- Homework 2 220EE3310Document3 pagesHomework 2 220EE3310bharathNo ratings yet
- EV Quantum NumberDocument29 pagesEV Quantum Numbermuhammad.tamzid.islamNo ratings yet
- RT Solutions-18!12!2011 XIII VXY Paper I Code ADocument17 pagesRT Solutions-18!12!2011 XIII VXY Paper I Code Avishal110085No ratings yet
- cs250-SolvedDocument5 pagescs250-SolvedHoang The AnhNo ratings yet
- Balance Algebraico IIDocument8 pagesBalance Algebraico IILilianaCatalinaMejiaLizcanoNo ratings yet
- Problem 2 SolutionDocument2 pagesProblem 2 SolutionMohammad AqelNo ratings yet
- Answers To Selected Problems: Chopra: Prentice-Hall PAGES JUL. 19, 2000 14:45 ICC Oregon (503) 221-9911Document16 pagesAnswers To Selected Problems: Chopra: Prentice-Hall PAGES JUL. 19, 2000 14:45 ICC Oregon (503) 221-9911MakaraSoyNo ratings yet
- Solution To Exercises 1.1: Answers: X 2.5375 + 1.9080i R 0.8161 - 0.0000iDocument8 pagesSolution To Exercises 1.1: Answers: X 2.5375 + 1.9080i R 0.8161 - 0.0000iGera VillaNo ratings yet
- 817 832 PDFDocument16 pages817 832 PDFMakaraSoyNo ratings yet
- Text 11Document4 pagesText 11gverma9461No ratings yet
- Answer Key: Special TEST-2 1 5 - 0 1 - 2 0 1 2Document15 pagesAnswer Key: Special TEST-2 1 5 - 0 1 - 2 0 1 2vishal110085No ratings yet
- Text 11Document4 pagesText 11gverma9461No ratings yet
- Part A Part A Part A Parta - Chemistry Chemistry Chemistry ChemistryDocument30 pagesPart A Part A Part A Parta - Chemistry Chemistry Chemistry Chemistryishika_94No ratings yet
- Fourier TableDocument4 pagesFourier Tablehomer1_210788No ratings yet
- U U J I I: Homework #1 Solutions For The Course of Photonics EngineeringDocument1 pageU U J I I: Homework #1 Solutions For The Course of Photonics EngineeringAppleWangAceNo ratings yet
- Quiz I: If The Reaction (3) Is Rate-LimitingDocument3 pagesQuiz I: If The Reaction (3) Is Rate-LimitingAsmZziz OoNo ratings yet
- College of Engineering Putrajaya Campus Test Ii SEMESTER 2 2009 / 2010Document4 pagesCollege of Engineering Putrajaya Campus Test Ii SEMESTER 2 2009 / 2010zawirNo ratings yet
- Problem Set Solutions CHAPTER 2, Levine, Quantum Chemistry, 5Document8 pagesProblem Set Solutions CHAPTER 2, Levine, Quantum Chemistry, 5Marcos ViníciusNo ratings yet
- Ejercicios Primera y Segunda Ley 13-08Document5 pagesEjercicios Primera y Segunda Ley 13-08Alexander ramosNo ratings yet
- Mackay Benzene For HW2Document31 pagesMackay Benzene For HW2raymundo pantraniNo ratings yet
- AIPMT Exam Solved Question Paper 2011Document36 pagesAIPMT Exam Solved Question Paper 2011cbsestudymaterialsNo ratings yet
- 4 MetamechDocument10 pages4 MetamechMatheus OliveiraNo ratings yet
- Meta/Mech: A Knowledge Based System For Formation EvaluationDocument10 pagesMeta/Mech: A Knowledge Based System For Formation EvaluationTripoli ManoNo ratings yet
- 2 Chemistry - Chemical Kinetics - 2 60 SolutionsDocument10 pages2 Chemistry - Chemical Kinetics - 2 60 SolutionsPRUTHVINo ratings yet
- Aiats Aipmt 2015 Test-2Document9 pagesAiats Aipmt 2015 Test-2Juhi NeogiNo ratings yet
- Index: 1 Input File 2 Solution History 3 MainDocument43 pagesIndex: 1 Input File 2 Solution History 3 MainMón Quà Vô GiáNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Prediction Variance YDocument1 pagePrediction Variance Ygillnumber22No ratings yet
- Prediction Variance YDocument1 pagePrediction Variance Ygillnumber22No ratings yet
- Coefficient of DeterminationDocument1 pageCoefficient of Determinationgillnumber22No ratings yet
- DW SeperationDocument4 pagesDW Seperationgillnumber22No ratings yet
- T With n-2Document1 pageT With n-2gillnumber22No ratings yet
- Proof 8againDocument1 pageProof 8againgillnumber22No ratings yet
- 10.67 Follow UpDocument1 page10.67 Follow Upgillnumber22No ratings yet
- The Sum of The Eigen Values of A Square Matrix Is Its TraceDocument1 pageThe Sum of The Eigen Values of A Square Matrix Is Its Tracegillnumber22No ratings yet
- Follow Up 11 3Document2 pagesFollow Up 11 3gillnumber22No ratings yet
- Integral WeibullDocument1 pageIntegral Weibullgillnumber22No ratings yet
- The Sum of The Eigenvalues of A Square Matrix Is Its TraceDocument5 pagesThe Sum of The Eigenvalues of A Square Matrix Is Its Tracegillnumber22No ratings yet
- Proof 7 AgainDocument1 pageProof 7 Againgillnumber22No ratings yet
- 1? I Get Confused I Guess From Previous Discussions That Is Binomial But I Don't See That It Is Similar Since P Is Larger Then 1Document1 page1? I Get Confused I Guess From Previous Discussions That Is Binomial But I Don't See That It Is Similar Since P Is Larger Then 1gillnumber22No ratings yet
- Proof 7Document1 pageProof 7gillnumber22No ratings yet
- CO CO Coefficient CO CO CoefficientDocument7 pagesCO CO Coefficient CO CO Coefficientgillnumber22No ratings yet
- Proof 2Document1 pageProof 2gillnumber22No ratings yet
- Difference T and Z-TableDocument1 pageDifference T and Z-Tablegillnumber22No ratings yet