Professional Documents
Culture Documents
Group 1 + 2 Trends & Reactions
Group 1 + 2 Trends & Reactions
Uploaded by
Andy PrendergastCopyright:
Available Formats
You might also like
- The S-Block ElementsDocument7 pagesThe S-Block ElementsSteveMathewKuruvillaNo ratings yet
- CAIE Chemistry A-Level: 10: Group 2Document6 pagesCAIE Chemistry A-Level: 10: Group 2ahumanbeinginearthNo ratings yet
- The S-Block Elements - WatermarkDocument26 pagesThe S-Block Elements - Watermarkakhil01ajNo ratings yet
- Group 2 Past PapersDocument13 pagesGroup 2 Past PapersShiloh FrederickNo ratings yet
- Chemistry Seminar: Done By: Lamha HarisDocument7 pagesChemistry Seminar: Done By: Lamha HarisLamha HarisNo ratings yet
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Shujaat SiddiquiNo ratings yet
- S - and P-Block ElementsDocument8 pagesS - and P-Block Elementssameenrashid410No ratings yet
- 10 Chemistry ABS 2Document5 pages10 Chemistry ABS 2Aryan GuptaNo ratings yet
- S Block ElementsDocument11 pagesS Block Elements19ucha023 19ucha023No ratings yet
- To Investigate The Period 3 OxideDocument2 pagesTo Investigate The Period 3 OxideSandy Ing Xiang Chee33% (3)
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Mildred MunatsiNo ratings yet
- CHEM IA #4 (Fixed)Document4 pagesCHEM IA #4 (Fixed)phillipssillinaNo ratings yet
- S Block ElementsDocument35 pagesS Block ElementsAdrita KakotyNo ratings yet
- Hydrogen Is The of The Periodic Table.: First ElementDocument5 pagesHydrogen Is The of The Periodic Table.: First Elementnofodic311No ratings yet
- Group 2Document21 pagesGroup 2Zareen KidwaiNo ratings yet
- Chemistry and Water, Solution Full NoteDocument39 pagesChemistry and Water, Solution Full NotenifbaeNo ratings yet
- Metals and Non-MetalsDocument29 pagesMetals and Non-MetalsemillaNo ratings yet
- S Block ElementsDocument6 pagesS Block Elementskeshavjain7No ratings yet
- Alkali MetalsDocument13 pagesAlkali Metalsmunzeerlubna4No ratings yet
- Hydroxides of .. Group 1 Metals Group 2Document2 pagesHydroxides of .. Group 1 Metals Group 2Victor ChanNo ratings yet
- Acid Bases SummaryDocument8 pagesAcid Bases Summaryibraheemgamer786No ratings yet
- Xi Chem 9. Hydrogen Anil HssliveDocument5 pagesXi Chem 9. Hydrogen Anil HssliveAnonymous 9uu04elNo ratings yet
- Lecture 6-Group 1 & 2Document34 pagesLecture 6-Group 1 & 2Kumar KeshavNo ratings yet
- Catabas - Double Replacement ReactionDocument3 pagesCatabas - Double Replacement Reactionapi-233267698No ratings yet
- METALS AND NON METALS NoteDocument38 pagesMETALS AND NON METALS NoteYusuf AkinyooyeNo ratings yet
- 25 HydrogenDocument53 pages25 HydrogenAbdul MateenNo ratings yet
- Group 2 ElementsDocument9 pagesGroup 2 ElementsSumaya TraoreNo ratings yet
- Hydrogen: Position in The Periodic TableDocument5 pagesHydrogen: Position in The Periodic TableAura WaxNo ratings yet
- Acids, Bases and SaltsDocument8 pagesAcids, Bases and Saltsaakashb1918No ratings yet
- Group 16: Classification of OxidesDocument14 pagesGroup 16: Classification of OxidesP. PARIS KATHERINE REBECCAH BCMBC2019No ratings yet
- Alkaline Earth MetalsDocument12 pagesAlkaline Earth Metalselango achamNo ratings yet
- Group 2Document32 pagesGroup 2irnihafizan6812No ratings yet
- Group 2Document15 pagesGroup 2Behzod ShoraimovNo ratings yet
- 10 - Group 2Document20 pages10 - Group 2Soma Chowdhury RosyNo ratings yet
- CIE AS ChemistryDocument21 pagesCIE AS ChemistryiceforgamingNo ratings yet
- Reactivity of Families of Elements in The Periodic Tabl1Document6 pagesReactivity of Families of Elements in The Periodic Tabl1Runo OmughelliNo ratings yet
- HANDOUT OF INORGANIC CHEMISTRY (Federal) - 2Document15 pagesHANDOUT OF INORGANIC CHEMISTRY (Federal) - 2mahnoors571No ratings yet
- Inorganic Chemistry Is The Branch ofDocument30 pagesInorganic Chemistry Is The Branch ofNurhidayahAzizNo ratings yet
- Qualitative AnalysisDocument90 pagesQualitative AnalysisMahesh100% (1)
- AnionsDocument90 pagesAnionsAnish RaoNo ratings yet
- 2017s Block ElementsDocument16 pages2017s Block ElementsAnkit LakshyaNo ratings yet
- Spodumene Lial (Sio) Petalite (Lial (Si O), Lepidolite K (Li, Al) (Al, Si, RB) O (F, Oh)Document16 pagesSpodumene Lial (Sio) Petalite (Lial (Si O), Lepidolite K (Li, Al) (Al, Si, RB) O (F, Oh)dannyNo ratings yet
- InorganicDocument6 pagesInorganicb52352986No ratings yet
- Ch-2 Acids, Bases &saltsDocument77 pagesCh-2 Acids, Bases &saltsakhil.jNo ratings yet
- Groups 2&7Document16 pagesGroups 2&7Kalel WilsonNo ratings yet
- Group 2 & 7 CHML PropertiesDocument2 pagesGroup 2 & 7 CHML PropertiesΜοχαμέδ ΑτταρNo ratings yet
- Water Technology Part 2Document69 pagesWater Technology Part 2ronnie199250% (2)
- Group 2Document19 pagesGroup 2Muhammad KalimNo ratings yet
- Study Guide Chem 110 Exam 1Document6 pagesStudy Guide Chem 110 Exam 1Khusbu PatelNo ratings yet
- Ch-3 Metals and Non-MetalsDocument85 pagesCh-3 Metals and Non-Metalsakhil.jNo ratings yet
- Chemistry Inorganic ProjectDocument11 pagesChemistry Inorganic ProjectemillaNo ratings yet
- Answering QuestionDocument4 pagesAnswering QuestionUli Lumban GaolNo ratings yet
- Chemical Reactions of Group IV Elements (3) CompressedDocument9 pagesChemical Reactions of Group IV Elements (3) CompressedTahera McsweenNo ratings yet
- S Block ElementsDocument4 pagesS Block ElementssubkitsNo ratings yet
- All Reactions - PadhleDocument18 pagesAll Reactions - Padhlerakshitham603No ratings yet
- Acid Bases and Salts Igcse Chemistry 0620Document15 pagesAcid Bases and Salts Igcse Chemistry 0620Aminah ShahzadNo ratings yet
- Chemistry Topic 4Document10 pagesChemistry Topic 4manahilimrNo ratings yet
- Advances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookFrom EverandAdvances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookArmando J. L. PombeiroRating: 5 out of 5 stars5/5 (1)
Group 1 + 2 Trends & Reactions
Group 1 + 2 Trends & Reactions
Uploaded by
Andy PrendergastOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Group 1 + 2 Trends & Reactions
Group 1 + 2 Trends & Reactions
Uploaded by
Andy PrendergastCopyright:
Available Formats
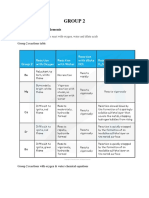
Group 1 & 2: Trends and Reactions
Trend/Reaction Compound/Species
Nitrate
Group 1
Very difficult to decompose. Although, forms NITRITE
Group 2
Thermal stability
Carbonate
Hydroxide Nitrate
Carbonate
Solubility
Sulphate
Element
Water
Element
Halogens
Ba(NO3)2 most difficult to decompose Forms NO2, OXIDE and O2 BaCO3 most Very difficult to difficult to decompose. decompose Forms OXIDE and CO2 CsOH most Ba(OH)2 most soluble soluble All very soluble All very soluble Although larger Although larger cations less so cations less so Na-Cs very All insoluble soluble Although Although MgCO3 is to a lithium very degree insouble BaSO4 least All soluble soluble Be/MgSO4 will form aqueous solution Forms Forms HYDROXIDE HYDROXIDE and H2 and H2 Oxide formed with Mg and steam Forms IONIC Forms IONIC SALT: MX SALT: MX2 Small cations Small cations with IODINE with IODINE form more form more covalent covalent compounds compounds
You might also like
- The S-Block ElementsDocument7 pagesThe S-Block ElementsSteveMathewKuruvillaNo ratings yet
- CAIE Chemistry A-Level: 10: Group 2Document6 pagesCAIE Chemistry A-Level: 10: Group 2ahumanbeinginearthNo ratings yet
- The S-Block Elements - WatermarkDocument26 pagesThe S-Block Elements - Watermarkakhil01ajNo ratings yet
- Group 2 Past PapersDocument13 pagesGroup 2 Past PapersShiloh FrederickNo ratings yet
- Chemistry Seminar: Done By: Lamha HarisDocument7 pagesChemistry Seminar: Done By: Lamha HarisLamha HarisNo ratings yet
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Shujaat SiddiquiNo ratings yet
- S - and P-Block ElementsDocument8 pagesS - and P-Block Elementssameenrashid410No ratings yet
- 10 Chemistry ABS 2Document5 pages10 Chemistry ABS 2Aryan GuptaNo ratings yet
- S Block ElementsDocument11 pagesS Block Elements19ucha023 19ucha023No ratings yet
- To Investigate The Period 3 OxideDocument2 pagesTo Investigate The Period 3 OxideSandy Ing Xiang Chee33% (3)
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Mildred MunatsiNo ratings yet
- CHEM IA #4 (Fixed)Document4 pagesCHEM IA #4 (Fixed)phillipssillinaNo ratings yet
- S Block ElementsDocument35 pagesS Block ElementsAdrita KakotyNo ratings yet
- Hydrogen Is The of The Periodic Table.: First ElementDocument5 pagesHydrogen Is The of The Periodic Table.: First Elementnofodic311No ratings yet
- Group 2Document21 pagesGroup 2Zareen KidwaiNo ratings yet
- Chemistry and Water, Solution Full NoteDocument39 pagesChemistry and Water, Solution Full NotenifbaeNo ratings yet
- Metals and Non-MetalsDocument29 pagesMetals and Non-MetalsemillaNo ratings yet
- S Block ElementsDocument6 pagesS Block Elementskeshavjain7No ratings yet
- Alkali MetalsDocument13 pagesAlkali Metalsmunzeerlubna4No ratings yet
- Hydroxides of .. Group 1 Metals Group 2Document2 pagesHydroxides of .. Group 1 Metals Group 2Victor ChanNo ratings yet
- Acid Bases SummaryDocument8 pagesAcid Bases Summaryibraheemgamer786No ratings yet
- Xi Chem 9. Hydrogen Anil HssliveDocument5 pagesXi Chem 9. Hydrogen Anil HssliveAnonymous 9uu04elNo ratings yet
- Lecture 6-Group 1 & 2Document34 pagesLecture 6-Group 1 & 2Kumar KeshavNo ratings yet
- Catabas - Double Replacement ReactionDocument3 pagesCatabas - Double Replacement Reactionapi-233267698No ratings yet
- METALS AND NON METALS NoteDocument38 pagesMETALS AND NON METALS NoteYusuf AkinyooyeNo ratings yet
- 25 HydrogenDocument53 pages25 HydrogenAbdul MateenNo ratings yet
- Group 2 ElementsDocument9 pagesGroup 2 ElementsSumaya TraoreNo ratings yet
- Hydrogen: Position in The Periodic TableDocument5 pagesHydrogen: Position in The Periodic TableAura WaxNo ratings yet
- Acids, Bases and SaltsDocument8 pagesAcids, Bases and Saltsaakashb1918No ratings yet
- Group 16: Classification of OxidesDocument14 pagesGroup 16: Classification of OxidesP. PARIS KATHERINE REBECCAH BCMBC2019No ratings yet
- Alkaline Earth MetalsDocument12 pagesAlkaline Earth Metalselango achamNo ratings yet
- Group 2Document32 pagesGroup 2irnihafizan6812No ratings yet
- Group 2Document15 pagesGroup 2Behzod ShoraimovNo ratings yet
- 10 - Group 2Document20 pages10 - Group 2Soma Chowdhury RosyNo ratings yet
- CIE AS ChemistryDocument21 pagesCIE AS ChemistryiceforgamingNo ratings yet
- Reactivity of Families of Elements in The Periodic Tabl1Document6 pagesReactivity of Families of Elements in The Periodic Tabl1Runo OmughelliNo ratings yet
- HANDOUT OF INORGANIC CHEMISTRY (Federal) - 2Document15 pagesHANDOUT OF INORGANIC CHEMISTRY (Federal) - 2mahnoors571No ratings yet
- Inorganic Chemistry Is The Branch ofDocument30 pagesInorganic Chemistry Is The Branch ofNurhidayahAzizNo ratings yet
- Qualitative AnalysisDocument90 pagesQualitative AnalysisMahesh100% (1)
- AnionsDocument90 pagesAnionsAnish RaoNo ratings yet
- 2017s Block ElementsDocument16 pages2017s Block ElementsAnkit LakshyaNo ratings yet
- Spodumene Lial (Sio) Petalite (Lial (Si O), Lepidolite K (Li, Al) (Al, Si, RB) O (F, Oh)Document16 pagesSpodumene Lial (Sio) Petalite (Lial (Si O), Lepidolite K (Li, Al) (Al, Si, RB) O (F, Oh)dannyNo ratings yet
- InorganicDocument6 pagesInorganicb52352986No ratings yet
- Ch-2 Acids, Bases &saltsDocument77 pagesCh-2 Acids, Bases &saltsakhil.jNo ratings yet
- Groups 2&7Document16 pagesGroups 2&7Kalel WilsonNo ratings yet
- Group 2 & 7 CHML PropertiesDocument2 pagesGroup 2 & 7 CHML PropertiesΜοχαμέδ ΑτταρNo ratings yet
- Water Technology Part 2Document69 pagesWater Technology Part 2ronnie199250% (2)
- Group 2Document19 pagesGroup 2Muhammad KalimNo ratings yet
- Study Guide Chem 110 Exam 1Document6 pagesStudy Guide Chem 110 Exam 1Khusbu PatelNo ratings yet
- Ch-3 Metals and Non-MetalsDocument85 pagesCh-3 Metals and Non-Metalsakhil.jNo ratings yet
- Chemistry Inorganic ProjectDocument11 pagesChemistry Inorganic ProjectemillaNo ratings yet
- Answering QuestionDocument4 pagesAnswering QuestionUli Lumban GaolNo ratings yet
- Chemical Reactions of Group IV Elements (3) CompressedDocument9 pagesChemical Reactions of Group IV Elements (3) CompressedTahera McsweenNo ratings yet
- S Block ElementsDocument4 pagesS Block ElementssubkitsNo ratings yet
- All Reactions - PadhleDocument18 pagesAll Reactions - Padhlerakshitham603No ratings yet
- Acid Bases and Salts Igcse Chemistry 0620Document15 pagesAcid Bases and Salts Igcse Chemistry 0620Aminah ShahzadNo ratings yet
- Chemistry Topic 4Document10 pagesChemistry Topic 4manahilimrNo ratings yet
- Advances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookFrom EverandAdvances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookArmando J. L. PombeiroRating: 5 out of 5 stars5/5 (1)