Professional Documents
Culture Documents
Term Symb
Term Symb
Uploaded by
한송이0 ratings0% found this document useful (0 votes)
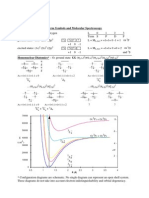
7 views1 pageThe document discusses term symbols and molecular spectroscopy. It provides the ground and excited state term symbols for oxygen atoms, with the ground state being (1s)2(2s)2(2p)4 and excited state being (1s)2(2s)2(2p)4. It also discusses the molecular orbital diagram and term symbols for the homonuclear diatomic molecule O2, showing the molecular orbital configurations and resulting molecular terms. Finally, it shows the potential energy curves for various electronic states of the O2 molecule.
Original Description:
Original Title
TermSymb
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses term symbols and molecular spectroscopy. It provides the ground and excited state term symbols for oxygen atoms, with the ground state being (1s)2(2s)2(2p)4 and excited state being (1s)2(2s)2(2p)4. It also discusses the molecular orbital diagram and term symbols for the homonuclear diatomic molecule O2, showing the molecular orbital configurations and resulting molecular terms. Finally, it shows the potential energy curves for various electronic states of the O2 molecule.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
7 views1 pageTerm Symb
Term Symb
Uploaded by
한송이The document discusses term symbols and molecular spectroscopy. It provides the ground and excited state term symbols for oxygen atoms, with the ground state being (1s)2(2s)2(2p)4 and excited state being (1s)2(2s)2(2p)4. It also discusses the molecular orbital diagram and term symbols for the homonuclear diatomic molecule O2, showing the molecular orbital configurations and resulting molecular terms. Finally, it shows the potential energy curves for various electronic states of the O2 molecule.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
Term Symbols and Molecular Spectroscopy
Atomic Term Symbols - Oxygen
ground state: (1s)2 (2s)2 (2p)4
+1
2
excited state: (1s) (2s) (2p)
L
0
1
2
3
Term S
P
D
F
L = ML,max = +1+1+ 0 -1 = 1 3P
-1
L = ML,max = +1+1+ 0 +0 = 2 1D
+1
-1
Homonuclear Diatomics* O2 ground state: KK (g,2s)2(*u ,2s)2(g,2pz)2(u,2p)4(*g ,2p)2
u
g
u
g
= +1+1-1-1+1-1 = 0
= +1+1-1-1+1+1 = 2
3 -
= +1+1-1-1+1-1 = 0
1 +
(g,2pz)2(u,2p)3(*g ,2p)3
= +1+1-1+1-1-1 = 0
3 +
(g,2pz)1(u,2p)4(*g ,2p)3
= +1+1-1+1+1-1 = 2
3 -
u + u + 3u
70000
O( 3P) + O( 1D)
8-
60000
3
u
50000
6 O( 3P) + O(3 P)
40000
3 +
u
(cm-1)
4 -
30000
eV
20000
1 +
g
1
g
3
g
10000
0
0.5
1.5
2 -
2.5
3.5
R ()
* Configuration diagrams are schematic. No single diagram can represent an open shell system.
These diagrams do not take into account electron indistinguishability and orbital degeneracy.
You might also like
- Solutions To Problems, Capitulo 2 LevenspielDocument6 pagesSolutions To Problems, Capitulo 2 LevenspielAlexander Gonzalez Romero75% (4)
- Chapter 11 InorgDocument12 pagesChapter 11 InorgMauritiusFeliciano100% (6)
- Term SymbDocument1 pageTerm Symb丁健佑No ratings yet
- B.Sc. SEM-VI Us06Cche22 Inorganic Chemistry Unit-3 (A) Term Symbol (B) Electronic Spectra of Metal ComplexesDocument34 pagesB.Sc. SEM-VI Us06Cche22 Inorganic Chemistry Unit-3 (A) Term Symbol (B) Electronic Spectra of Metal ComplexesChaithraMallu100% (1)
- Aufbaus and Hunds RuleDocument36 pagesAufbaus and Hunds RuleMirjeta ZymeriNo ratings yet
- Jordanova Forma I Jordanova BazaDocument2 pagesJordanova Forma I Jordanova BazajusescrustNo ratings yet
- Chem 373 - Lecture 14: Molecular Structure and The Born Oppenheimer ApproximationDocument19 pagesChem 373 - Lecture 14: Molecular Structure and The Born Oppenheimer ApproximationNuansak3No ratings yet
- BY Muhammad Humais Yamin: HomewoekDocument4 pagesBY Muhammad Humais Yamin: HomewoekHumais yaminNo ratings yet
- Motion in PlaneDocument12 pagesMotion in PlaneBindu MenonNo ratings yet
- Exposicion G.Alvear PDFDocument119 pagesExposicion G.Alvear PDFdaniel ramosNo ratings yet
- MathsDocument5 pagesMathsCharlie PinedoNo ratings yet
- Inverse Laplace TransformDocument26 pagesInverse Laplace TransformIñigo SorianoNo ratings yet
- Ormulario Y Constantes Físicas: FormularioDocument2 pagesOrmulario Y Constantes Físicas: FormularioEmilio PérezNo ratings yet
- Fungsi Gelombang Orbital Dan Bilangan Kuantum Atom: Tugas Kimia Fisika IDocument10 pagesFungsi Gelombang Orbital Dan Bilangan Kuantum Atom: Tugas Kimia Fisika IReviaNo ratings yet
- 7 LaplaceDocument16 pages7 LaplaceAldiansyahRudyNo ratings yet
- Term SymbolDocument20 pagesTerm SymbolRirin Zarlina100% (1)
- Electronic Spectroscopy 1Document62 pagesElectronic Spectroscopy 1api-372459780% (5)
- Trabajo Práctico de Inducción MatemáticaDocument32 pagesTrabajo Práctico de Inducción MatemáticaMarcos T.No ratings yet
- Some Inequalities Concerning Smarandache's FunctionDocument9 pagesSome Inequalities Concerning Smarandache's FunctionMia AmaliaNo ratings yet
- Gerretsen InequalityDocument6 pagesGerretsen InequalityMoti LevyNo ratings yet
- OXIDATION-REDUCTION REACTIONS (Redox Reactions) (SJ, P. 316)Document29 pagesOXIDATION-REDUCTION REACTIONS (Redox Reactions) (SJ, P. 316)Jon Bisu Debnath0% (1)
- Electronic Spectra of TM ComplexesDocument42 pagesElectronic Spectra of TM ComplexesAmalia AnggreiniNo ratings yet
- Lecture-12 - 16-11-22Document20 pagesLecture-12 - 16-11-22Alkit SharmaNo ratings yet
- Quantum Mechanical Model of An AtomDocument46 pagesQuantum Mechanical Model of An AtomCrystle Hailey FernandezNo ratings yet
- Unit 2 Chapter 9 Answers PDFDocument12 pagesUnit 2 Chapter 9 Answers PDFBexxysassNo ratings yet
- Ecercises For Section 1.2Document11 pagesEcercises For Section 1.2Leni MaarlinaNo ratings yet
- FactorialDocument4 pagesFactorialAnonymous kcAhyrfN2l100% (1)
- l18 Hunds Max Multiplicity Pauli Exclusion Practice QuestionDocument16 pagesl18 Hunds Max Multiplicity Pauli Exclusion Practice Questiondevendra singhNo ratings yet
- (A305) Otomatik Kontrol Ders Notu (Slayt)Document27 pages(A305) Otomatik Kontrol Ders Notu (Slayt)Mücahit Ezel100% (1)
- Ch7 Laplace TransformDocument15 pagesCh7 Laplace TransformumerNo ratings yet
- Statistics Solution PEjedEiDocument44 pagesStatistics Solution PEjedEigjkjjj784No ratings yet
- 7 Laplace - PD SerentakDocument15 pages7 Laplace - PD SerentakAldiansyahRudyNo ratings yet
- Laplace ProblemsDocument39 pagesLaplace ProblemsHussain JiffryNo ratings yet
- Term Symbols & Spitting of Terms - Transition Metal ComplexesDocument16 pagesTerm Symbols & Spitting of Terms - Transition Metal Complexesramukaka100% (3)
- 9 LaplaceDocument16 pages9 LaplaceYudhie RahmanNo ratings yet
- Fhsc1134 Ioc Chapter 1Document32 pagesFhsc1134 Ioc Chapter 1Tie Teck HoeNo ratings yet
- Slater Condon Rules DerivationDocument7 pagesSlater Condon Rules DerivationSean MacfoyNo ratings yet
- Feedback Laboratory AssignmentDocument5 pagesFeedback Laboratory AssignmentmultisporkyNo ratings yet
- Periodic Table PDFDocument5 pagesPeriodic Table PDFSingh PrinceNo ratings yet
- Funciones de Transferencia de Sistemas LinealesDocument6 pagesFunciones de Transferencia de Sistemas LinealespelusokidoNo ratings yet
- For Homework AssignmentDocument48 pagesFor Homework AssignmentyinglvNo ratings yet
- ON THE CUBIC RESIDUES NUMBERS AND k-POWER COMPLEMENT NUMBERSDocument6 pagesON THE CUBIC RESIDUES NUMBERS AND k-POWER COMPLEMENT NUMBERSRyanEliasNo ratings yet
- Chem 373 - Lecture 20: Complex Atomic SpectraDocument27 pagesChem 373 - Lecture 20: Complex Atomic SpectraNuansak3No ratings yet
- Eamcet Key 09Document61 pagesEamcet Key 09saisuchandanNo ratings yet
- LÓ¡pl J ØV¡ X Fé¡ LS: Advanced ChemistryDocument22 pagesLÓ¡pl J ØV¡ X Fé¡ LS: Advanced ChemistrysandipanNo ratings yet
- Ramanujan's "Lost" Notebook. II. @-Function Expansions: @,, (Z, Q) 2q114 Sin Z FiDocument13 pagesRamanujan's "Lost" Notebook. II. @-Function Expansions: @,, (Z, Q) 2q114 Sin Z Fiappleamirali186No ratings yet
- Advanced Problems.Document4 pagesAdvanced Problems.Charlie PinedoNo ratings yet
- CHM 101 - 2Document8 pagesCHM 101 - 2enocholadeinde2004No ratings yet
- Formule EconometrieDocument1 pageFormule EconometrieDaniela BatrinceaNo ratings yet
- Aufbau-Perodic Table02Document2 pagesAufbau-Perodic Table02infinitumeyesNo ratings yet
- Problema Dinamica 41Document3 pagesProblema Dinamica 41Cipar ClauNo ratings yet
- Unit-VII Laplace Transforms: Properties of Laplace Transforms: L. Linearity Property: IfDocument21 pagesUnit-VII Laplace Transforms: Properties of Laplace Transforms: L. Linearity Property: IfRanjan NayakNo ratings yet
- Math3as Activities Istidlal MebarkiDocument4 pagesMath3as Activities Istidlal MebarkiAlicherif Benaissa100% (1)
- MOT Part 2Document24 pagesMOT Part 2suka11blyatNo ratings yet
- And X: LKN - OK.Qjn 1Document5 pagesAnd X: LKN - OK.Qjn 1Charlie PinedoNo ratings yet
- Practica 2 ExDocument3 pagesPractica 2 Exjulian aruquipaNo ratings yet
- Handout For Russell-Saunders Coupling PDFDocument12 pagesHandout For Russell-Saunders Coupling PDFAditiNo ratings yet
- A General Result On The Smarandache Star FunctionDocument13 pagesA General Result On The Smarandache Star FunctionDon HassNo ratings yet
- Analytic Geometry: Graphic Solutions Using Matlab LanguageFrom EverandAnalytic Geometry: Graphic Solutions Using Matlab LanguageNo ratings yet
- Sweet Baby Dresses in Crochet: 4 Dresses in Sizes Newborn to 24 Months, with Matching AccessoriesFrom EverandSweet Baby Dresses in Crochet: 4 Dresses in Sizes Newborn to 24 Months, with Matching AccessoriesRating: 4 out of 5 stars4/5 (2)