Professional Documents
Culture Documents
Embryonic Development of The Heart
Embryonic Development of The Heart
Uploaded by
Matthew RyanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Embryonic Development of The Heart
Embryonic Development of The Heart
Uploaded by
Matthew RyanCopyright:
Available Formats
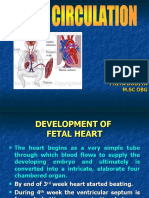
EMBRYONIC DEVELOPMENT OF THE HEART The heart is the first functioning organ in the embryo; its first pulsatile
movements begin during the third week after conception. This early development of the heart is essen- tial to the rapidly growing embryo as a means of circulating nutrients and removing waste products. Most of the development of the heart and blood vessels occurs be- tween the third and eighth weeks of embryonic life. The developing heart begins as two endothelial tubes that fuse into a single tubular structure. The early heart structures develop as the tubular heart elongates and forms alternate dilations and constrictions. A single atrium and ventricle along with the bulbus cordis develop first. This is followed by formation of the truncus arteriosus and the sinus venosus, a large venous sinus that receives blood from the embryo and developing placenta (Fig. 2624). The early pulsatile movements of the heart begin in the sinus venosus and move blood out of the heart by way of the bulbus cordis, truncus arteriosus, and aortic arches. A differential growth rate in the early cardiac struc- tures, along with fixation of the heart at the venous and arterial ends, causes the tubular heart to bend over on it- self. As the heart bends, the atrium and the sinus venosus come to lie behind the bulbus cordis, truncus arteriosus, and ventricle. This looping of the primitive heart results in the hearts alignment in the left side of the chest with the atrium located behind the ventricle. Malrotation during formation of the ventricular loop can cause various malpositions, such as dextroposition of the heart. The embryonic heart undergoes further development as partitioning of the chambers occurs. Partitioning of the AV canal, atrium, and ventricle begins in the fourth week and essentially is complete by the fifth week. The separation of the heart begins as tissue bundles, called the endocardial cushions, form in the midportion of the dorsal and ventral walls of the heart in the region of the AV canal and begin to grow inward. Until the separation begins, a single AV canal exists between the atria and the ventricles. As the endocardial cushions enlarge, they meet and fuse to form separate right and
left AV canals (Fig. 26-25). The mitral and tricuspid valves develop in these canals. The endocar- dial cushions also contribute to formation of parts of the atrial and ventricular septum. Defects in endocardial cush- ion formation can result in atrial and ventricular septal defects, complete AV canal defects, and anomalies of the mitral and tricuspid valves. Compartmentalization of the ventricles begins with the growth of the interventricular septum from the floor of the ventricle moving upward toward the endocardial cushions. Fusion of the endocardial cushions with the interventricular septum usually is completed by the end of the seventh week. Partitioning of the atrial septum is more complex and occurs in two stages, beginning with the formation of a thin, crescent-shaped membrane called the septum primum that emerges from the anterosuperior portion of the heart and grows toward the endocardial cushions, leaving an opening called the foramen primum between its lower edge and the endocardial cushions. A second membrane, called the septum secundum, also begins to grow from the upper wall of the atrium on the right side of the septum primum. As this membrane grows toward the endocardial cushions, it gradually overlaps an opening in the upper part of the septum primum, forming an oval opening with a flap-type valve called the foramen ovale (see Fig. 26-25). The upper part of the septum primum gradually disappears; the re- maining part becomes the valve of the foramen ovale. The foramen ovale forms a communicating channel between the two upper chambers of the heart. This opening, which closes shortly after birth, allows blood from the umbilical vein to pass directly into the left heart, bypassing the lungs. An ostium secundum defect, which is one type of atrial septal defect, is thought to result from excessive absorption of the septum primum. To complete the transformation into a four-chambered heart, provision must be made for separating the blood pumped from the right side of the heart, which is to be diverted into the pulmonary circulation, from the blood pumped from the left side of the heart, which is to be pumped to the systemic circulation. This separation of blood flow is accomplished by developmental changes in the outflow channels of the tubular heart, the bulbus cordis and the truncus arteriosus, which undergo spiral twisting and vertical partitioning (Fig. 26-26). As these vessels spiral and divide, the location of the aorta becomes posterior and to the right of the pulmonary artery. Impaired spiraling dur- ing this stage of development can lead to defects such as transposition of the great vessels.
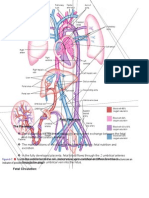
In the process of forming a separate pulmonary trunk and aorta, a vessel called the ductus arteriosus develops. This vessel, which connects the pulmonary artery and the aorta, allows blood entering the pulmonary trunk to be shunted into the aorta as a means of bypassing the lungs. Like the foramen ovale, the ductus arteriosus usually closes shortly after birth. FETAL AND PERINATAL CIRCULATION The fetal circulation is different anatomically and physiologically than the postnatal circulation. Before birth, oxygenation of blood occurs by way of the placenta, and after birth, it occurs by way of the lungs. The fetus is maintained in a low-oxygen state (PO2, 30 to 35 mm Hg; 60% to 70% saturation).7072 To compensate, fetal cardiac out- put is higher than at any other time in life (400 to 500 mL/ kg/minute). Also, the pulmonary vessels in the fetus are markedly constricted owing to the fluid-filled lungs and the heightened hypoxic stimulus for vasoconstriction that is present in the fetus. As a result, blood flow through the lungs is less than at any other time in life. In the fetus, blood enters the circulation through the umbilical vein and returns to the placenta by way of the two umbilical arteries (Fig. 26-27). A vessel called the ductus venosum allows blood from the umbilical vein to bypass the hepatic circulation and pass directly into the inferior vena cava. From the inferior vena cava, blood flows into the right atrium and then moves through the foramen ovale into the left atrium. It then passes into the left ventricle and is ejected into the ascending aorta to perfuse the head and upper extremities. In this way, the best- oxygenated blood from the placenta is used to perfuse the brain. At the same time, venous blood from the head and upper extremities returns to the right side of the heart by way of the superior vena cava, moves into the right ventricle, and is ejected into the pulmonary artery. Because of the very high pulmonary vascular resistance that is pre- sent, blood ejected into the pulmonary artery gets diverted through the ductus arteriosus into the descending aorta. This blood perfuses the lower extremities and is returned to the placenta by way of the umbilical arteries. At birth, the infant takes its first breath and switches from placental to
pulmonary oxygenation of the blood. The most dramatic alterations in the circulation after birth are the elimination of the low-resistance placental vascular bed and the marked pulmonary vasodilation that is produced by initiation of ventilation. The pressure in the pulmonary circulation and the right side of the heart falls as fetal lung fluid is replaced by air and as lung expansion de- creases the pressure transmitted to the pulmonary blood vessels. With lung inflation, the alveolar oxygen tension increases, causing reversal of the hypoxemia-induced pulmonary vasoconstriction of the fetal circulation. Cord clamping and removal of the low-resistance placental circulation produce an increase in systemic vascular resistance and a resultant increase in left ventricular pressure. The resultant decrease in right atrial pressure and increase in left atrial pressure produce closure of the foramen ovale. Reversal of the fetal hypoxemic state also produces constriction of ductal smooth muscle, contributing to closure of the ductus arteriosus. The foramen ovale and the ductus arteriosus normally close within the first day of life, effectively separating the pulmonary and systemic circulations. After the initial precipitous fall in pulmonary vascular resistance, a more gradual decrease in pulmonary vascular resistance is related to regression of the medial smooth muscle layer in the pulmonary arteries. During the first 2 to 9 weeks of life, gradual thinning of the smooth muscle layer results in further decreases in pulmonary vascu- lar resistance. By the time a healthy, term infant is several weeks old, the pulmonary vascular resistance has fallen to adult levels. Several factors, including prematurity, alveolar hypoxia, lung disease, and congenital heart defects, may affect postnatal pulmonary vascular development.72 If an infant is born prematurely, the smooth muscle layers of the pulmonary vasculature may develop incompletely or regress in a shorter period. Much of the development of the smooth muscle layer in the pulmonary arterioles occurs during the latter part of gestation; as a result, infants who are born pre- maturely have less medial smooth muscle. These infants follow the same pattern of smooth muscle regression, but because less muscle exists, the muscle layer may regress in a shorter period. The pulmonary vascular smooth muscle in premature infants also may be less responsive to hypoxia. For these reasons, a premature infant may demonstrate a larger decrease in pulmonary vascular resistance and a re-
sultant shunting of blood from the aorta through the ductus arteriosus to the pulmonary artery within hours of birth. Hypoxia during the first days of life may also delay or prevent the normal decrease in pulmonary vascular resistance. During this period, the pulmonary arteries remain highly reactive and can constrict in response to hypoxia, acidosis, hyperinflation of the alveoli, and hypothermia. Alveolar hypoxia is one of the most potent stimuli of pulmonary vasoconstriction and pulmonary hypertension in the neonate.
Lower Airway Infections Lower airway infections produce air trapping with pro- longed expiration. Wheezing results from bronchospasm, mucosal inflammation, and edema. The child presents with increased expiratory effort, increased respiratory rate, and wheezing. If the infection is severe, there also are marked intercostal retractions and signs of impending respiratory failure.
Acute bronchiolitis is a viral infection of the lower air- ways, most commonly caused by the respiratory syncytial virus.76,78,80 Other viruses, such as parainfluenza-3 virus and some adenoviruses, as well as mycoplasma, also are causative. The infection produces inflammatory obstruction of the small airways and necrosis of the cells lining the lower airways. It occurs during the first 2 years of life, with a peak incidence between 3 to 6 months of age. The source of infection usually is a family member with a minor respiratory illness. Older children and adults tolerate bronchiolar edema much better than infants and do not manifest the clinical picture of bronchiolitis. Because the resistance to airflow in a tube is related to the fourth power of the radius, even minor swelling of bronchioles in an infant can produce profound changes in airflow.
Most affected infants in whom bronchiolitis develops have a history of a mild upper respiratory tract infection. These symptoms usually last several days and may be ac- companied by fever and diminished appetite. There is then a gradual development of respiratory distress, characterized by a wheezy cough, dyspnea, and irritability. The infant usually is able to take in sufficient air but has trouble exhaling it. Air becomes trapped in the lung distal to the site of obstruction and interferes with gas exchange.
Hypoxemia and, in severe cases, hypercapnia may develop. Airway obstruction may produce air trapping and hyperinflation of the lungs or collapse of the alveoli. Infants with acute bronchiolitis have a typical appearance, marked by breathlessness with rapid respirations, a distressing cough, and retractions of the lower ribs and sternum. Crying and feeding exaggerate these signs. Wheezing and crackles may or may not be present, depending on the degree of airway obstruction. In infants with severe airway obstruction, wheezing decreases as the airflow diminishes. Usually, the most critical phase of the disease is the first 48 to 72 hours. Cyanosis, pallor, listlessness, and sudden diminution or absence of breath sounds indicate impending respiratory failure. The characteristics of bronchiolitis are described in Table 30-2. Infants with respiratory distress usually are hospitalized. Treatment is supportive and includes administration of humidified oxygen to relieve hypoxia. Elevation of the head facilitates respiratory movements and avoids airway compression. Handling is kept at a minimum to avoid tiring. Because the infection is viral, antibiotics are not effective and are given only for a secondary bacterial infection. Dehydration may occur as the result of increased insensible water losses because of the rapid respiratory rate and feeding difficulties, and measures to ensure adequate hydration are needed. Recovery usually begins after the first 48 to 72 hours and usually is rapid and complete. Signs of Impending Respiratory Failure Respiratory problems of infants and small children often are of sudden origin, and recovery usually is rapid and complete. Children are at risk for the development of air- way obstruction and respiratory failure resulting from obstructive disorders or lung infection. The child with epiglottitis is at risk for airway obstruction. The child with bronchiolitis is at risk for respiratory failure resulting from impaired gas exchange. Children with impending respiratory failure due to airway or lung disease have rapid breathing; exaggerated use of the accessory muscles; retractions, which are more pronounced in the child than in the adult because of more compliant chest; nasal flaring; and grunting during expiration. Signs of Respiratory Distress and Impending Respiratory Failure in the Infant and Small Child:
Severe increase in respiratory effort, including severe retractions or grunting, decreased chest movement Cyanosis that is not relieved by administration of oxygen (40%) Heart rate of 150 beats per minute or greater or brady- cardia Very rapid breathing (rate 60 per minute in the new- born to 6 months or above 30 per minute in children 6 months to 2 years) Very depressed breathing (rate 20 per minute or below) Retractions of the supraclavicular area, sternum, epigastric and intercostal spaces. Extreme anxiety and agitation
Fatigue Decreased level of consciousness
CYSTIC FIBROSIS Cystic fibrosis (CF), which is the major cause of severe chronic respiratory disease in children, is an autosomal recessive disorder involving fluid secretion in the exocrine glands in the epithelial lining of the respiratory, gastro- intestinal, and reproductive tracts.4446 In addition to chronic respiratory disease, CF is manifested by pancreatic exocrine deficiency and elevation of sodium chloride in the sweat. Nasal polyps, sinus infections, pancreatitis, and cholelithiasis also are common. Excessive loss of sodium in the sweat predisposes young children to salt depletion episodes. Most males with cystic fibrosis have congenital bilateral absence of the vas deferens with azoospermia.
Cystic fibrosis is caused by mutations in a single gene on the long arm of chromosome 7 that encodes for the cystic fibrosis transmembrane regulator (CFTR), which functions as a chloride (Cl) channel in epithelial cell membranes. The disease affects approximately 30,000 children and adults in the United States, and more than 10 million persons are asymptomatic carriers of the defective gene. The gene is rare in African blacks and Asians. Homozygotes (i.e., persons with two defective genes) have all or substantially all of the clinical symptoms of the disease, compared with heterozygotes, who are carriers of the disease but have no recognizable symptoms. Although a large number of mutations in the CFTR gene have been identified, only 22 have been identified with any degree of frequency. The most common and first identified mutation, which involves a three base pair deletion that codes for phenylalanine, accounts for 70% of CF cases in whites. Mutations in the CFTR gene render the epithelial membrane relatively impermeable to the chloride ion. The impact on transport function is relatively tissue specific. In the sweat glands, the concentration of sodium (Na+) and Cl secreted into the lumen of the gland remains unaffected, whereas the reabsorption of Cl through the CFTR and accompanying reabsorption of Na+ in the ducts of the gland fail to occur. This accounts for the high con- centration of NaCl in the sweat of persons with CF.48 In the normal airway epithelium, Cl is secreted into airway lumen through the CFTR. The impaired transport of Cl ultimately leads to a series of secondary events, including in- creased absorption of Na+ and water from the airways into the blood. This lowers the water content of the mucociliary blanket coating the respiratory epithelium, causing it to become more viscid. The resulting dehydration of the mucus layer leads to defective mucociliary function and accumulation of viscid secretions that obstruct the airways and predispose to recurrent pulmonary infections. Similar transport abnormalities and pathophysiologic events take place in the pancreatic and biliary ducts and in the vas deferens in males. Respiratory manifestations of CF are caused by an accumulation of viscid mucus in the bronchi, impaired mucociliary clearance, and lung infections. Chronic bronchiolitis and bronchitis are the initial lung manifestations, but after months and years, structural changes in the bronchial wall lead to bronchiectasis. In addition to airway obstruction, the basic genetic defect that occurs with CF predisposes to chronic infection with a surprising small
number of organisms, the most common being Pseudomonas aeruginosa, followed by Staphylococcus aureus, Haemophilus influenza, and Stenotrophomonas maltophilia.46 Soon after birth, initial infection with bacterial pathogens occurs and is associated with an excessive neutrophilic inflammatory response that seems to occur independent of in- fection. Recent studies suggest that because of the Cl channel defect, the epithelium consumes more oxygen than normal; thus, creating an anaerobic environment. The normally aerobic P. aeruginosa responds to the change by assuming a mucoid phenotype that is resistant to phagocytosis by neutrophils. Even with intensive antibiotic regimens, the mucoid P. aeruginosa cannot be eradicated, probably because of poor penetration of antibiotics into anaerobic sputum plugs and development of antibiotic- resistant strains of the organism. Pancreatic function is abnormal in approximately 80% to 90% of affected persons.49 Steatorrhea, diarrhea, and abdominal pain and discomfort are common. In the newborn, meconium ileus may cause intestinal obstruction. The degree of pancreatic involvement is highly variable n some children, the defect is relatively mild, and in others, the involvement is severe and impairs intestinal absorption. In addition to exocrine pancreatic insufficiency, hyperglycemia may occur, especially after 10 years of age, when approximately 8% of persons with cystic fibrosis develop diabetes mellitus. Early diagnosis and treatment are important in delaying the onset and severity of chronic illness in children with CF. Diagnosis is based on the presence of respiratory and gastrointestinal manifestations typical of cystic fibrosis, a history of cystic fibrosis in a sibling, or a positive new- born screening test. Confirmatory laboratory tests include the sweat test, assessment of bioelectrical properties of respiratory epithelia by measurement of transepithelial potential differences in the nasal membrane, and genetic tests for CFTR gene mutations. The sweat test, using pilocarpine iontophoresis to collect the sweat followed by chemical analysis of its chloride content, remains the standard approach to diagnosis. Newborns with cystic fibrosis have elevated blood levels of immunoreactive trypsinogen, presumably because of secretory obstruction in the pancreas. Newborn screening consists of a test for determination of immunoreactive trypsinogen. The test can be done on blood spots collected for routine newborn screening tests.
At present, there are no approved treatments for correcting the genetic defects in CF or to reverse the ion transport abnormalities associated with the dysfunctional CFTR. Thus, treatment measures are directed to- ward slowing the progression of secondary organ dysfunction and sequelae such as chronic lung infection and pancreatic insufficiency. They include the use of antibiotics to prevent and manage infections; the use of chest physical therapy (chest percussion and postural drainage) and mucolytic agents to prevent airway obstruction; and pancreatic enzyme replacement and nutritional therapy. Routine laboratory evaluations are key to assessing pulmonary function and response to therapeutic interventions. These studies include radiologic examinations, pulmonary function testing, and microbiologic cultures of respiratory secretions. Appropriate antibiotic therapy directed against bacterial pathogens isolated from the respiratory tract is an essential component in the management of CF lung disease. Antibiotics are initially used to prevent colonization with P. aeruginosa; they are used as maintenance therapy once the airways are colonized with P. aeruginosa and other or- ganisms such as S. aureus; and they are administered as aggressive treatment during acute exacerbations of pulmonary symptoms caused by infections. To avoid adverse effects and to obtain high airway concentrations, the inhalation route is often used. Indications for oral antibiotics include the presence of respiratory tract symptoms and identification of pathogenic organisms in respiratory tract cultures. Intravenous antibiotics are used for progressive and unrelenting symptoms. The abnormal viscosity of airway secretions is attributed largely to the presence of polymorphonuclear white blood cells and their degradation products. A purified re- combinant human deoxyribonuclease (rhDNase), an enzyme that breaks down these products, has been developed. Clinical trials have shown that the drug, which is administered by inhalation, can improve pulmonary symptoms and reduce the frequency of respiratory exacerbations. Although many persons benefit from the therapy, the drug is costly, and recommendations for its use are evolving. Up to 90% of patients with CF have complete loss of exocrine pancreas function and inadequate digestion of fats and proteins. They require diet adjustment, pancreatic enzyme replacement, and supplemental vitamins and minerals. Many individuals with CF have higher than normal caloric need
because of the increased work of breathing and perhaps because of the increased metabolic activity related to the basic defect. Pancreatic enzyme dosage and product type are individualized for each patient. Enteric-coated, pH- sensitive enzyme microspheres are available. A low-fat, high-protein, high-calorie diet was generally recommended in the past. With the advent of improved pancreatic enzyme products, however, normal amounts of fat in the diet are usually tolerated and preferred. Progress of the disease is variable. Improved medical management has led to longer survival. Today, nearly 40% of people with CF are 18 years of age or older. Lung trans- plantation is being used as a treatment for persons with end-stage lung disease. Current hopes reside in research that would make gene therapy a feasible alternative for persons with the disease.
You might also like
- DocuNotes Clinical Pocket Guide To Effective ChartingDocument203 pagesDocuNotes Clinical Pocket Guide To Effective ChartingShirlyn Ares Navarro96% (47)
- Breath Sounds Incredibly EasyDocument4 pagesBreath Sounds Incredibly EasyMatthew Ryan100% (3)
- CVS Embryology Questions and Study Guide - Quizlet Flashcards by Hugo - OxfordDocument5 pagesCVS Embryology Questions and Study Guide - Quizlet Flashcards by Hugo - OxfordAzizNo ratings yet
- Your Breath On Yoga: The Anatomy & Physiology of Breathing for Teachers and Students of YogaFrom EverandYour Breath On Yoga: The Anatomy & Physiology of Breathing for Teachers and Students of YogaNo ratings yet
- Circulatory System: A Tutorial Study GuideFrom EverandCirculatory System: A Tutorial Study GuideRating: 5 out of 5 stars5/5 (3)
- Nursing Management of Seizures and EpilepsyDocument36 pagesNursing Management of Seizures and EpilepsyMatthew Ryan100% (6)
- The Brigham Renal Board Review CourseDocument2,195 pagesThe Brigham Renal Board Review CourseHydralazine100% (6)
- Zy24r1ayjqzfi0yjuvi0mayl PDFDocument2 pagesZy24r1ayjqzfi0yjuvi0mayl PDFAnonymous 3OYSY7r100% (1)
- Fetal Circulation .Document21 pagesFetal Circulation .namulema AngellaNo ratings yet
- Fetal CorrectionDocument9 pagesFetal Correctionprabhdeep kaurNo ratings yet
- Fetal CirculationDocument2 pagesFetal CirculationCJ PorrasNo ratings yet
- Formation of The HeartDocument12 pagesFormation of The HeartAlyssa VillanoNo ratings yet
- Fetal CirculationDocument62 pagesFetal CirculationRed Williams100% (4)
- Development of The HeartDocument4 pagesDevelopment of The HeartMatthew GiscombeNo ratings yet
- Development of The Heart and Blood VesselsDocument10 pagesDevelopment of The Heart and Blood VesselsAbigail ChristisnNo ratings yet
- Fetal Circulation in The WombDocument2 pagesFetal Circulation in The WombChristineAlaNo ratings yet
- Embryology CvsDocument31 pagesEmbryology CvskarthickbozzNo ratings yet
- Fetal CirculationDocument4 pagesFetal CirculationNishaThakuriNo ratings yet
- Progenitor Heart Cells Lie in The Epiblast, Immediately Adjacent To TheDocument15 pagesProgenitor Heart Cells Lie in The Epiblast, Immediately Adjacent To TheJehangir AllamNo ratings yet
- The Cardiac Cycle 2Document7 pagesThe Cardiac Cycle 2Abigail ChristisnNo ratings yet
- Circulatory System - Part 2 4-8-14 For BBDocument23 pagesCirculatory System - Part 2 4-8-14 For BBroman7dbNo ratings yet
- Update Fetal Circulation - MDM AmyDocument10 pagesUpdate Fetal Circulation - MDM AmyNana Yunus100% (1)
- Development of CVSDocument51 pagesDevelopment of CVSalnifeady9No ratings yet
- Fetal Circulation and Circulatory Changes at Birth - Pulei - 2024-05-03Document36 pagesFetal Circulation and Circulatory Changes at Birth - Pulei - 2024-05-03shemobaigwa79No ratings yet
- FETALDocument9 pagesFETALGunaNo ratings yet
- Development of Cardiovascular SystemDocument8 pagesDevelopment of Cardiovascular Systemwmdpr4x64fNo ratings yet
- Fetal Circulation FinalDocument21 pagesFetal Circulation FinalpriyaNo ratings yet
- Congenital Heart DiseasesDocument55 pagesCongenital Heart DiseasesFaheem BasharatNo ratings yet
- Fetal CirculationDocument5 pagesFetal CirculationJaime PorscheNo ratings yet
- Cardiovascular System Embryology Applied Aspects: Preceptor Prof S S KothariDocument109 pagesCardiovascular System Embryology Applied Aspects: Preceptor Prof S S KothariBir Singh100% (1)
- Fetal CirculationDocument2 pagesFetal CirculationTracy88% (8)
- Embryology of The HeartDocument65 pagesEmbryology of The Heartnisha24No ratings yet
- Adaption of Infants To Extra-Uterine LifeDocument10 pagesAdaption of Infants To Extra-Uterine LifeJulienne QuintaoNo ratings yet
- Fetal CirculationDocument3 pagesFetal Circulationyusrinastiti100% (1)
- Fetal CirculationDocument20 pagesFetal CirculationMuhyeeSalaIdjadNo ratings yet
- Embryology of Heart and LungDocument7 pagesEmbryology of Heart and Lungavinash dhameriyaNo ratings yet
- Heart Development - WikipediaDocument42 pagesHeart Development - WikipediaABHINABA GUPTANo ratings yet
- Anatomy Tutorials - Cardiac DevelopmentDocument6 pagesAnatomy Tutorials - Cardiac DevelopmentSamantha HohNo ratings yet
- Development of The HeartDocument19 pagesDevelopment of The HeartYusuf UmarNo ratings yet
- Development of The Cardiovascular System - TeachMeAnatomyDocument5 pagesDevelopment of The Cardiovascular System - TeachMeAnatomyNashrah Nashrah (22043)No ratings yet
- Development of Vasculature: Compare and Contrast Vasculogenesis and AngiogenesisDocument5 pagesDevelopment of Vasculature: Compare and Contrast Vasculogenesis and AngiogenesisKingNo ratings yet
- Septum Formation in The VentriclesDocument43 pagesSeptum Formation in The VentriclesAhsan IslamNo ratings yet
- Unit 3 Development of The HeartDocument12 pagesUnit 3 Development of The HeartJack TomarNo ratings yet
- Cvs Development by DR ZaighamDocument21 pagesCvs Development by DR ZaighamZaigham HammadNo ratings yet
- Cardiovascular Question JACKDocument1 pageCardiovascular Question JACKLeng BunthaiNo ratings yet
- Fetal Circulation Anatomy and Physiology of Fetal Circulation Umbilical CordDocument3 pagesFetal Circulation Anatomy and Physiology of Fetal Circulation Umbilical CordbobtagubaNo ratings yet
- Operation of The Heart ValvesDocument10 pagesOperation of The Heart Valvesgadis_wawa267friendlyNo ratings yet
- DFF FFF AaaaaDocument5 pagesDFF FFF AaaaaffffffffNo ratings yet
- Jantung (The Heart)Document24 pagesJantung (The Heart)JoePutraNo ratings yet
- Cardiovascular System AathifDocument9 pagesCardiovascular System Aathifl881539No ratings yet
- ANA 208 Cardiovascular SystemDocument21 pagesANA 208 Cardiovascular Systemayomideolaniran14No ratings yet
- Embryology of CVSDocument63 pagesEmbryology of CVSAdan Iman100% (1)
- Embryology of Heart and Fetal CirculationDocument36 pagesEmbryology of Heart and Fetal CirculationRaviNo ratings yet
- Anatomy of The Cardiopulmonary SystemDocument19 pagesAnatomy of The Cardiopulmonary Systemmercygreat2014No ratings yet
- Fetal CirculationDocument39 pagesFetal CirculationjerlinNo ratings yet
- Formation of Blood and ArteriesDocument7 pagesFormation of Blood and ArteriesAlyssa VillanoNo ratings yet
- BV Circulatory Sys Lec 2020Document39 pagesBV Circulatory Sys Lec 2020fevkloeroanefmabanagNo ratings yet
- Cardiac Embryology 1Document18 pagesCardiac Embryology 1Natalia IhalauwNo ratings yet
- Fetal Circulation: Dr. Khush BakhtDocument29 pagesFetal Circulation: Dr. Khush BakhtKhush BakhtNo ratings yet
- Development of Heart - NewDocument34 pagesDevelopment of Heart - Newmanish2manish7No ratings yet
- Fetal CirculationDocument2 pagesFetal CirculationEric Gato100% (3)
- 13 - Development of CVS-I (Heart) - DR - GosaiDocument60 pages13 - Development of CVS-I (Heart) - DR - GosaiDr.B.B.GosaiNo ratings yet
- Congenital Heart DIseasesDocument3 pagesCongenital Heart DIseasesAshan BopitiyaNo ratings yet
- Fetal CirculationDocument23 pagesFetal CirculationVaibhav BhadageNo ratings yet
- GlomerulonephritisDocument8 pagesGlomerulonephritisMatthew Ryan100% (1)
- Acute and Chronic PyelonephritisDocument7 pagesAcute and Chronic PyelonephritisMatthew Ryan100% (1)
- Acute Respiratory Distress SyndromeDocument9 pagesAcute Respiratory Distress SyndromeMatthew Ryan100% (2)
- Fetal Perfusion/Congenital Heart DefectsDocument13 pagesFetal Perfusion/Congenital Heart DefectsMatthew RyanNo ratings yet
- Newborn RespirationDocument3 pagesNewborn RespirationMatthew RyanNo ratings yet
- IV InfiltrationDocument4 pagesIV InfiltrationMatthew Ryan100% (1)
- Wound Management With Excessive DrainageDocument6 pagesWound Management With Excessive DrainageMatthew RyanNo ratings yet
- Nursing Wound AssessmentDocument3 pagesNursing Wound AssessmentMatthew RyanNo ratings yet
- Neural Tube DefectsDocument2 pagesNeural Tube DefectsMatthew RyanNo ratings yet
- Assessing Apical PulseDocument5 pagesAssessing Apical PulseMatthew Ryan100% (1)
- Health Promotion and MaintenanceDocument18 pagesHealth Promotion and MaintenanceMatthew Ryan100% (1)
- Unit 8: Pain Assessment and ManagementDocument24 pagesUnit 8: Pain Assessment and ManagementMatthew RyanNo ratings yet
- Pediatric Fluid and Electrolyte AlterationsDocument51 pagesPediatric Fluid and Electrolyte AlterationsMatthew Ryan100% (1)
- Pregnancy at Risk Preexisting ConditionsDocument39 pagesPregnancy at Risk Preexisting ConditionsMatthew RyanNo ratings yet
- Chapter 8 Nursing Care During Labor and Pain ManagementDocument34 pagesChapter 8 Nursing Care During Labor and Pain ManagementMatthew Ryan100% (1)
- Neonatal Temp Reg CeDocument9 pagesNeonatal Temp Reg CeMatthew RyanNo ratings yet
- The Human Genome ProjectDocument22 pagesThe Human Genome ProjectAashianaThiyam100% (1)
- Pattern Recognition Receptors (PRRS) : Rossana Zaru, University of Dundee, UkDocument4 pagesPattern Recognition Receptors (PRRS) : Rossana Zaru, University of Dundee, UkFree Escort ServiceNo ratings yet
- Cocaine Long Term EffectsDocument3 pagesCocaine Long Term Effectsaysha_chaudhry5520No ratings yet
- Amblyopia eDocument3 pagesAmblyopia eIeien MuthmainnahNo ratings yet
- Evolution of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Cyclooxygenase (COX) Inhibition and BeyondDocument31 pagesEvolution of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Cyclooxygenase (COX) Inhibition and BeyondAlexandru SavaNo ratings yet
- Myocardial Infarction Pathophysiology Schematic DiagramDocument3 pagesMyocardial Infarction Pathophysiology Schematic Diagramnursing concept mapsNo ratings yet
- Adult Med HXDocument2 pagesAdult Med HXapi-3868874No ratings yet
- Seminar On Cementum: Shreya Das Intern Department of PeriodontiaDocument57 pagesSeminar On Cementum: Shreya Das Intern Department of Periodontiashreya dasNo ratings yet
- Necrotizing SialometaplasiaDocument33 pagesNecrotizing SialometaplasiaJessica GreenNo ratings yet
- CRIMINALISTICSDocument66 pagesCRIMINALISTICSJhezzie GutierrezNo ratings yet
- English Pedagogical Module 1: Where Are You From?Document32 pagesEnglish Pedagogical Module 1: Where Are You From?juanandres2978100% (1)
- EpidemiologyDocument10 pagesEpidemiologyMUTHUKUMARANNo ratings yet
- Chapter 11 - Mutation: Study QuestionsDocument7 pagesChapter 11 - Mutation: Study QuestionsMahmOod GhNo ratings yet
- Immunologhy Lab Report 3 - MURAT GENÇ-2021652244Document10 pagesImmunologhy Lab Report 3 - MURAT GENÇ-2021652244Murat GençNo ratings yet
- Colorectal Cancer PresentationDocument30 pagesColorectal Cancer PresentationfaikaNo ratings yet
- What Are The Functions of ProteinsDocument12 pagesWhat Are The Functions of ProteinsQue TenederoNo ratings yet
- Pex 09 06Document4 pagesPex 09 06Illich Ramirez Tanta100% (2)
- H1d - Microcytic Anemias ChartDocument3 pagesH1d - Microcytic Anemias ChartqselmmNo ratings yet
- RDRP Inhibitors Insilico ACSOmegaDocument11 pagesRDRP Inhibitors Insilico ACSOmegaKübra KahveciNo ratings yet
- Personalised Medicine in PsychiatryDocument58 pagesPersonalised Medicine in PsychiatryAttaullah khanNo ratings yet
- Clinical StudyDocument9 pagesClinical Studymeltwithsnow163.comNo ratings yet
- 9700 w16 QP 41Document28 pages9700 w16 QP 41Chiaw YeeNo ratings yet
- Pathogenesis of Infectious DiseaseDocument31 pagesPathogenesis of Infectious Diseaserockmitrill100% (8)
- Integrating Biomedical Ontologies - Obr-Scolio Ontology: Vanja LukovićDocument6 pagesIntegrating Biomedical Ontologies - Obr-Scolio Ontology: Vanja LukovićUbiquitous Computing and Communication JournalNo ratings yet
- Presentation 1Document57 pagesPresentation 1Brian BeeNo ratings yet
- 15 3Document4 pages15 3Fernanda Carvalho Machado CortesNo ratings yet
- Brian Perry: Cosmetic MicrobiologyDocument3 pagesBrian Perry: Cosmetic MicrobiologyNukiAdelaNo ratings yet
- Standardization of RatesDocument57 pagesStandardization of RatesmengyingNo ratings yet