Professional Documents
Culture Documents
Ida Kusuma - Acid & Base Application in Pharmaceutical
Ida Kusuma - Acid & Base Application in Pharmaceutical
Uploaded by
zuzuzazaziziOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ida Kusuma - Acid & Base Application in Pharmaceutical
Ida Kusuma - Acid & Base Application in Pharmaceutical
Uploaded by
zuzuzazaziziCopyright:
Available Formats
Ida Kusuma PE-5
Acid & Base Application in Pharmaceutical
Acid and base are one of the most common chemical substances we found in our daily life. Many substances such as vinegar, ammonia and amino acids, are classified as either an acid or a base. One of the most important properties of acids and bases is their reaction with each other, which is neutralization.1 The reaction is as simple as the production of salt and water when acids reacted with bases. This property of acids and bases is also used in pharmaceuticals, namely in healing some diseases caused by the disruption of acid-base balance in human body. An acid is simply a substance which reacts with a base. Acids generally have a tart and sour taste. Common examples of acid are acetic acid in vinegar, citric acid in lemon, and ammonia in our pee. Aqueous acids have a pH under 7, with the acidity increasing the lower the pH. According to the Brnsted-Lowry definition, an acid is a substance that can act as a proton donor. The proton mentioned is usually in the form of H+ ion. An acid is also a substance that accepts a pair of electrons from another substance or also known as electron pair acceptor. There are lots of pharmaceutical ingredients in a medicine that is categorized as an acid. One of the most noted applications of acid is in the famous-headache-reliefmedicine called aspirin. The active metabolite in aspirin is known as acetylsalicylic acid. Acetylsalicylic acid is often used as an analgesic to relieve minor aches and as antipyretic to reduce fever. It works by reducing substances in the body that cause the pain, fever, and inflammation.2 Like any other medications, there will always be some adverse affect in the use of aspirin. The use of aspirin adequately will relieve the pain. However, if used excessively or inadequately, aspirin will not heal the pain, but adding the damage to human body. One of the damages is gastrointestinal bleeding. Aspirin use has been shown to increase the risk of gastrointestinal bleeding.3 Even though aspirin is coated by some enteric coating, it did not seem to reduce the risk. Another method that is used to prevent the problem is buffering. Buffered aspirin works by preventing the
Pharmaceutical Chemistry
Ida Kusuma PE-5
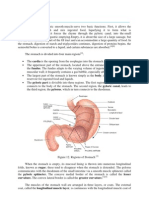
concentration of aspirin in the stomach walls. The buffering agent used in aspirin is usually magnesium oxide (MgO).4 Bases are basically the opposite of acids. Bases have a somewhat bitter taste and have a soapy and slick feel. Common examples of base are in the soap, detergent, and also milk. Aqueous bases have a pH above 7, with basicity increasing the higher the pH. A base is, according to the Brnsted-Lowry definition, a substance that can accept hydrogen ions (protons). A base is also a substance that donates a pair of valence electrons. Base is also referred as alkali. The example of the application of base in pharmaceutical is in the use of antacid. Antacids are medications that increase the pH balance in the stomach.5 Antacids are capable in treating a number of symptoms, including heartburn and gastritis. Heartburn is burning chest pain or discomfort that occurs when stomach acid leaks out of the stomach and into the esophagus. The acid then irritates the surface of the esophagus. This event then causes the distinct burning sensation in the chest.6 Antacids work in two ways. They can either make a coat of protective barrier against stomach acid on the surface of esophagus or produce gel on the stomach surface, which will then help to stop the acid leaking up into the esophagus, thus preventing the symptoms of heartburn. Antacids work by neutralizing the acid in human stomach and relieving the pain caused by the acid. Normally, the acid pH in human stomach is about 2 or 3. Trouble may start when the pH drops below those numbers. Some of the excess acid in the stomach will be neutralized by antacid until the pH in the stomach rises back to normal.7 Reference: [1] Brady, James E., Senese, Frederick A., Jerpersen, Neil D. 2009. Chemistry. Fifth Edition. Asia: John Wiley & Sons Pte. Ltd. [2] http://www.drugs.com/aspirin.html, 5 September 2012; 3.52 pm [3] http://www.sciencedaily.com/releases/2011/09/110912104830.htm, 5 September 2012;
3.52 pm [4] http://wiki.answers.com/Q/What_is_the_buffering_agent_in_buffered_aspirin, 5 September 2012; 3.52 pm [5] http://gerd.emedtv.com/antacids/antacids.html, 6 September 2012; 3.52 pm
Pharmaceutical Chemistry
Ida Kusuma PE-5 [6], [7] http://www.nhs.uk/conditions/antacid-medicines/Pages/Definition.aspx, 6 September 2012; 3.52 pm
Pharmaceutical Chemistry
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- DLL For Chemistry - Week 1Document2 pagesDLL For Chemistry - Week 1Jetz Hontimara Regio100% (3)
- Upstream PenicillinDocument6 pagesUpstream Penicillinzuzuzazazizi0% (1)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Category 022 - Cement Concrete ProductsDocument12 pagesCategory 022 - Cement Concrete Productsshahrilzainul77100% (1)
- Chapter 5 ExerciseDocument11 pagesChapter 5 ExerciseSuriaraj KrishnanNo ratings yet
- Kami Export - Cameron Martin - The - Properties - of - Water - Worksheet - 1Document5 pagesKami Export - Cameron Martin - The - Properties - of - Water - Worksheet - 1Cameron Martin100% (1)
- MSDS PVCDocument3 pagesMSDS PVCechavarriNo ratings yet
- +vfbvfiv7h Hajt Bvfoyq7'y 6 Bu+v7hDocument1 page+vfbvfiv7h Hajt Bvfoyq7'y 6 Bu+v7hzuzuzazaziziNo ratings yet
- +vfcrfiv7h Hajt Crfoyq7'y 6 Bu+v7hDocument1 page+vfcrfiv7h Hajt Crfoyq7'y 6 Bu+v7hzuzuzazaziziNo ratings yet
- +vfbvfiv7h Hajt Bvfoyq7'y 6 Bu+v7hDocument1 page+vfbvfiv7h Hajt Bvfoyq7'y 6 Bu+v7hzuzuzazaziziNo ratings yet
- LM, SFMM, Sfi, SFJH Hehjm, SF MM, Sfoym, Sfjhoy 6 Bulm, SFJHDocument1 pageLM, SFMM, Sfi, SFJH Hehjm, SF MM, Sfoym, Sfjhoy 6 Bulm, SFJHzuzuzazaziziNo ratings yet
- LM, SFB, Sfi, SFJH Hehjm, SF B, Sfoym, Sfjhoy 6 Bulm, SFJHDocument1 pageLM, SFB, Sfi, SFJH Hehjm, SF B, Sfoym, Sfjhoy 6 Bulm, SFJHzuzuzazaziziNo ratings yet
- LM, SFMM, Sfi, SFJH Hehjm, SF MM, Sfoym, Sfjhoy 6 Bulm, SFJHDocument1 pageLM, SFMM, Sfi, SFJH Hehjm, SF MM, Sfoym, Sfjhoy 6 Bulm, SFJHzuzuzazaziziNo ratings yet
- LM, SFMM, SFHM, SFJH Hehjm, SF MM, Sfigym, Sfjhigy 6 Bulm, SFJHDocument1 pageLM, SFMM, SFHM, SFJH Hehjm, SF MM, Sfigym, Sfjhigy 6 Bulm, SFJHzuzuzazaziziNo ratings yet
- LM, SFMM, Sfi, SFJH Hehjm, SF MM, Sfigym, Sfjhigy 6 Bulm, SFJHDocument1 pageLM, SFMM, Sfi, SFJH Hehjm, SF MM, Sfigym, Sfjhigy 6 Bulm, SFJHzuzuzazaziziNo ratings yet
- "Our Song": (Chorus:)Document2 pages"Our Song": (Chorus:)zuzuzazaziziNo ratings yet
- Young VolcanoesDocument1 pageYoung VolcanoeszuzuzazaziziNo ratings yet
- PharynxDocument2 pagesPharynxzuzuzazaziziNo ratings yet
- Layers of Gastrointestinal TractDocument2 pagesLayers of Gastrointestinal TractzuzuzazaziziNo ratings yet
- Stomach: Curvature. The Convex Lateral Border Is Called The Greater CurvatureDocument4 pagesStomach: Curvature. The Convex Lateral Border Is Called The Greater CurvaturezuzuzazaziziNo ratings yet
- Cerebrovit X-CelDocument36 pagesCerebrovit X-CelzuzuzazaziziNo ratings yet
- Chapter 16 - ReinforcementDocument12 pagesChapter 16 - ReinforcementrezadNo ratings yet
- Yamane M., Asahara Y.-Glasses For Photonics-CUP (2000)Document283 pagesYamane M., Asahara Y.-Glasses For Photonics-CUP (2000)EliudOrozcoNo ratings yet
- 05 LectureDocument98 pages05 LectureVishvraj DevmurariNo ratings yet
- Investigation of Thermo-Acoustic Excess Parameters of Binary Liquid Mixture Using Ultrasonic Non Destructive TechniqueDocument7 pagesInvestigation of Thermo-Acoustic Excess Parameters of Binary Liquid Mixture Using Ultrasonic Non Destructive TechniqueInternational Journal of Latest Research in Engineering and TechnologyNo ratings yet
- Introduction To Column Buckling: ©teaching Resource in Design of Steel StructuresDocument35 pagesIntroduction To Column Buckling: ©teaching Resource in Design of Steel Structureshemant_durgawaleNo ratings yet
- MCMT Unit-1 PPQDocument2 pagesMCMT Unit-1 PPQN Dhanunjaya Rao BorraNo ratings yet
- Table 1. Summary of Holding Times and Preservation For Liquid and Soil PHDocument3 pagesTable 1. Summary of Holding Times and Preservation For Liquid and Soil PHBenoitNo ratings yet
- Kentledge Design SpreadsheetDocument4 pagesKentledge Design SpreadsheetUtaya Kumar Veelmurugan100% (1)
- SP29 3 PDFDocument244 pagesSP29 3 PDFprasenjitsayantanNo ratings yet
- Xi Xii Chem Mcqs Nts and Past Papers UpdatedDocument118 pagesXi Xii Chem Mcqs Nts and Past Papers Updatedmuhammadmoosa6540No ratings yet
- Mokka Tips: Create A Unique Tactile Experience With Sappi's Touch Collection Release PapersDocument2 pagesMokka Tips: Create A Unique Tactile Experience With Sappi's Touch Collection Release Papersmanish singhalNo ratings yet
- Antibiotic Resistance in Wastewater BacteriaDocument14 pagesAntibiotic Resistance in Wastewater BacteriaraowaleedahmadNo ratings yet
- Set6ans 10Document4 pagesSet6ans 10AndrewNo ratings yet
- Cell Biology: General Facts of CellsDocument16 pagesCell Biology: General Facts of CellsbibhabNo ratings yet
- CP Violation and D Physics: Chris ParkesDocument23 pagesCP Violation and D Physics: Chris ParkesPraveen Dennis XavierNo ratings yet
- Thermal Measurements The Foundation of Fire Standards, ASTM, 2003Document199 pagesThermal Measurements The Foundation of Fire Standards, ASTM, 2003JheimyMarazNo ratings yet
- API 510 Final Model Exam-Open Book Page 1 of 6Document6 pagesAPI 510 Final Model Exam-Open Book Page 1 of 6jay2kay5793No ratings yet
- Rheovis - PU - 1341 - High ShearDocument2 pagesRheovis - PU - 1341 - High ShearYến HoàngNo ratings yet
- Magneos MCS 501Document4 pagesMagneos MCS 501wilber ticonaNo ratings yet
- HihDocument36 pagesHihBharati patil50% (2)
- US4668495Document5 pagesUS4668495Chemist Ahmed FoudaNo ratings yet
- Aluminium Dross Waste - Topics by Science - GovDocument198 pagesAluminium Dross Waste - Topics by Science - GovPramod Reddy TilletiNo ratings yet
- ch03 PDFDocument93 pagesch03 PDFSharuvindan NairNo ratings yet
- Syllabus PDFDocument75 pagesSyllabus PDFTehMarianNo ratings yet
- 0 7 PIA Module 15 (Gas Turbine Engine) Sub Module 15.7 (Exhaust)Document25 pages0 7 PIA Module 15 (Gas Turbine Engine) Sub Module 15.7 (Exhaust)soophiakNo ratings yet