Professional Documents
Culture Documents
Monobloc and Facial Bipartition Osteotomies
Monobloc and Facial Bipartition Osteotomies
Uploaded by
Kim SalahOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Monobloc and Facial Bipartition Osteotomies
Monobloc and Facial Bipartition Osteotomies
Uploaded by
Kim SalahCopyright:
Available Formats
Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
Monobloc and facial bipartition osteotomies
Ramon L. Ruiz, DMD, MDa,b,c,d,*, Timothy A. Turvey, DDSa,c, Paul S. Tiwana, DDS, MDa
a
Department of Oral and Maxillofacial Surgery, University of North Carolina at Chapel Hill, Brauer Hall, CB# 7450, Chapel Hill, NC 27599-7450, USA b Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA c Childrens Hospital of North Carolina, Chapel Hill, NC 27599, USA d University of North Carolina Craniofacial Center, Chapel Hill, NC 27599, USA
Maxillofacial surgery underwent a dramatic evolution as a direct result of the experience gained by surgeons who managed the facial injuries seen during the trench warfare of World War I [1]. The surgical techniques pioneered for the repair of injuries that involved the maxillofacial structures often were applied to the craniofacial skeleton and subsequently provided the foundation for newer procedures used in the reconstruction of congenital deformities. In the strictest sense, craniofacial surgical procedures are dened as those in which a transcranial approach is used for access to the upper facial skeleton. Despite this distinction, however, most craniofacial procedures represent an extension of the original scientic principles and traditional techniques of maxillofacial surgery. Tessier was the rst to describe an approach for the correction of the craniofacial anomalies associated with Crouzon and Apert syndromes using a Le Fort III osteotomy, which included a combined extracranial/transcranial approach, access via a coronal scalp ap, interpositional bone grafts for stabilization at the osteotomy sites, and an external xation device [24]. Subsequent modications [57] resulted in the development of the monobloc and facial bipartition procedures. More recently, the work of Posnick provides the most comprehensive description of the specic surgical techniques, additional technical renements, and clinical examples of ideal functional and esthetic results in the correction of total midface deciency associated with congenital deformities [813]. In North America, Posnicks numerous publications have established the role of the monobloc and facial bipartition procedures as viable reconstructive maneuvers in the craniofacial surgeons armamentarium. The purpose of this article is to provide the surgeon with an overview of the monobloc and facial bipartition procedures. The operative steps are described, with attention given to the indications for surgery and specic intraoperative, technical considerations.
Indications The craniofacial dysostosis syndromes (Crouzon, Apert, Pfeier, Saethre-Chotzen, and Carpenter) are inherited forms of craniosynostosis in which there is also extensive involvement of the sutures of the midfacial skeleton. In addition to the cranial vault dysmorphology that results from craniosynostosis (usually bilateral coronal), aected patients exhibit a characteristic total midface deciency that involves the orbits and maxilla. The surgical correction of the craniofacial anomalies of the craniofacial dysostosis syndromes requires at least three carefully sequenced stages of reconstruction [14,15]. Initially, release of the bilateral coronal synostosis with reshaping of the cranial vault is undertaken. The surgical
* Corresponding author. E-mail address: ruiz@med.unc.edu (R.L. Ruiz). 1061-3315/02/$ - see front matter 2002, Elsevier Science (USA). All rights reserved. PII: S 1 0 6 1 - 3 3 1 5 ( 0 1 ) 0 0 0 0 8 - 7
132
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
technique used is similar to that for patients with nonsyndromic bilateral coronal synostosis. Bifrontal craniotomy is combined with frontoorbital advancement and cranial vault reshaping. In the second stage of reconstruction, correction of the total midface deciency is undertaken. The goal of this operative procedure is to improve cranial vault morphology further, normalize intracranial volume, and address further the problem of inadequate orbital depth. The authors prefer to carry out this stage of the reconstructive sequence at approximately age 5 to 7, because the brain and cranioorbital structures have reached 80% to 90% of their adult size [16]. The operation can nalize the position of the orbits and shape of the forehead. Waiting until the permanent maxillary rst molars have erupted also decreases the likelihood of damaging these teeth and the developing second molars during the total midfacial osteotomy. It is at this stage in the reconstructive sequence of the craniofacial dysostosis syndromes that the monobloc and facial bipartition procedures may be applied. The exact timing of this operation also depends on the patients medical condition, functional neurologic concerns, and ophthalmologic situation. Patients with a marked degree of exorbitism are at risk for corneal injury, exposure keratitis, and disorders of ocular motility. Later in life, denitive orthognathic procedures are required to nalize the occlusion. The goal of second-stage reconstruction is to improve the contour and position of the orbits and forehead. Although the maxilla may be advanced into an ideal anteroposterior relationship with the mandible at the time of the monobloc osteotomy, it is not always possible to nalize the occlusion with this operation. It is predictable that in patients with craniofacial dysostosis there will be continued normal mandibular growth combined with decient maxillary development, which results in a signicant class III malocclusion. These patients require denitive orthognathic surgery to nalize the position of the lower face and occlusion. The surgical-orthodontic treatment is usually planned once growth of the maxilla and mandible is complete (1418 years of age). The most common application for the monobloc facial advancement procedure is in the nal reconstruction of the cranioorbital deformities associated with Crouzon syndrome. The monobloc osteotomy allows for advancement of the orbits, nasal complex, and maxilla as one unit. This procedure allows nal repositioning of the orbits while addressing the total midface deciency present in these patients. In cases in which the amount of forward movement required is dierent for the orbits than it is for the maxilla, the procedure allows for dierential movements, which is accomplished by further osteotomizing and recontouring the frontoorbital bandeau after the midface has been advanced. In cases in which the position and contour of the forehead and superior orbital rims are acceptable, a subcranial Le Fort III osteotomy may be used instead of the monobloc procedure. The decision regarding what type of osteotomy is carried out must be based on the specic skeletal dysmorphology and the anteroposterior position and contour of the frontoorbital region. Although there are similarities, the facial abnormalities associated with Apert syndrome generally are more pronounced and have less variation than those associated with Crouzon syndrome. Patients with Apert syndrome also demonstrate downslanting palpebral ssures and an increased facial width with orbital hypertelorism. In patients with Apert syndrome, secondstage reconstruction is carried out using a facial bipartition procedure combined with repeat cranial vault reshaping during childhood. Facial bipartition allows the surgeon to nalize the orbital contours and position, correct the orbital hypertelorism, and advance the middle face. The midline split and excision of a central fragment of bone permits forward rotation of the lateral orbits and the elimination of the at face appearance characteristic of Apert syndrome [8]. In these patients there may be an abnormally large frontal sinus, which must be managed along with the extradural dead space at the time of surgery. The standard approach involves cranialization, meticulous removal of the mucosal lining, and obliteration with autogenous material (pericranial ap, free fat graft, bone graft). The facial bipartition procedure also has found application in the reconstruction of the facial deformity associated with craniofrontonasal dysplasia and atypical midline cleft anomalies. In craniofrontonasal and frontonasal dysplasia, the typical skeletal dysmorphology is characterized by widening of the upper craniofacial segment and orbital hypertelorism (see Fig. 1). In general, the disproportionate skeletal growth is nonprogressive, and reconstruction of the midfacial skeleton should be delayed until the cranioorbital units are near skeletal maturity (57 years of age).
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
133
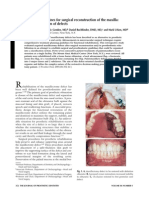
Fig. 1. (A) Preoperative view of a 6-year-old child with craniofrontonasal dysplasia. She underwent primary cranioorbtial decompression and release of bilateral coronal synostosis during infancy and returned for second-stage midfacial surgery. Surgical reconstruction consisted of a facial bipartition procedure with additional reshaping of the anterior cranial vault. (B) Severe orbital hypertelorism and widening of the upper craniofacial skeleton are noted. (C) Stereolithographic model of the same patient with proposed osteotomy sites. (D) Intraoperative view after exposure through a coronal ap, osteotomies, and disimpaction with complete mobilization of the midface. The area of midline ostectomy has been measured and marked. (E) Fragment of frontonasal bone excised measuring 27 mm. (F) Repositioned facial halves with initial xation. The interface is then recontoured using a surgical handpiece. (G) Intraoperative view after xation of repositioned frontal bones and the use of calcium phosphate cement for anterior cranial vault reshaping. (H) Postoperative frontal view 1 year after surgery.
134
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
Fig. 1 (continued )
Technique Coronal ap The coronal scalp incision is a versatile and cosmetically acceptable approach for access to the cranial vault, cranial base, forehead, nose, upper middle face, and orbits. With the use of this approach, inferior eyelid or transconjunctival access to the orbit in most cases is not necessary.
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
135
Fig. 1 (continued )
The incision is placed from one supraauricular area to the other, and the degree of skeletal exposure required for a given procedure dictates the inferior extent of the incisions. When access to the zygoma and infraorbital rims is necessary, the incisions must be extended further inferiorly. The hairline of the patient is a consideration in the placement of the incision. Although anterior extension at the midportion of the coronal ap may enhance ap retraction and access to the midface, the resulting scar subsequently may become obvious with male pattern baldness. The authors preference is to place the incision across the top of the head rather than carry it toward the forehead. The use of a postauricular coronal incision eliminates visible scars in
136
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
Fig. 1 (continued )
the preauricular area and decreases the risk to the frontal branch of the facial nerve in reoperated patients. Placement of this incision further posteriorly in the scalp is also benecial in children, in whom migration of the coronal scar may occur with growth. When secondary operations are performed, it is preferable to reincise through the original scar. Although it may be tempting to place the incision in a dierent location, consideration must be given to the eect of the previous scar on ap perfusion and wound healing. Use of a curved or sinusoidal (stealth) incision avoids a straight line scar and is particularly useful in patients with short hair. Initially, the proposed incision is injected with a diluted solution of 1% lidocaine with 1:200,000 epinephrine. This solution reduces bleeding and helps dissection along the subaponeurotic plane. Sterile saline may be injected freely into the subgaleal plane from the incision line to the forehead with the use of a spinal needle. The scalp has a rich vascular supply, so the incision is carried out in segments with the application of hemoclips. The authors preference is to begin with the bilateral supraauricular portions of the incision. Once the initial incision has been carried through the skin, two double-prong skin hooks are used to retract the wound edges outward. Blunt dissection with a surgical sponge or nger is then used through the loose subcutaneous connective tissue over each temporal extension to reach the temporalis fascia. Once the proper layer has been established, the superior portion of the coronal ap incision (superior temporal ridge to superior temporal ridge) is made. Bipolar electrocautery is used to obtain hemostasis, which has a minimal eect on the adjacent peripheral hair follicles. Once the pericranium is identied, a plane of dissection is established above it. Dissection proceeds rapidly and bloodlessly to the forehead in this supraperiosteal plane.
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
137
Once the anterior cranial vault is reached, the dissection is converted to the subperiosteal plane. An incision is carried through periosteum approximately 2 to 4 cm posterior to the superior orbital rims. It is critical to remain within the subperiosteal plane during dissection over the facial skeleton to avoid injury to the facial nerve. Bleeding from vessels that perforate the cranium can be controlled with bone wax. When monobloc facial advancement is undertaken, the authors prefer a modication in this technique that allows for the elevation of a large pericranial ap. A standard postauricular skin incision is made and dissection is started forward in the supragaleal plane. The posterior edge of the skin ap is undermined and the monopolar electrocautery is used to incise through the periosteum 1 to 2 cm behind the incision line. The pericranium is raised as an intact ap. The right and left margins of the ap are created along the superior temporal ridges and its anterior base is meticulously preserved to maintain an adequate blood supply. Subperiosteal dissection continues forward to expose the frontal bone and orbitozygomatic structures. Once the aps (coronal and pericranial) have been raised and they remain pedicled anteriorly, the pericranium is wrapped with a surgical sponge soaked with antibiotic-containing solution. This solution protects the pericranium and prevents desiccation. In infants and young children, care must be exercised when dissecting over open sutures, especially midline sutures, to avoid venous sinus hemorrhage or injury to the meninges. In older children who have undergone previous cranial vault surgery, full-thickness cranial defects may remain and make dissection and elevation of the coronal ap more complicated. Care also must be exercised when establishing a plane of dissection over the temporalis muscle. The natural plane of dissection is subgaleal. Within the region over the temporalis muscle, the plane should be deepened to the level of the muscle fascia (supercial layer of the deep temporal fascia). The temporoparietal fascia, which is supercial to the fascia of the temporalis muscle and is an extension of the supercial musculoaponeurotic system, invests the temporal branch of the facial nerve. Deepening the incision to the level of the temporalis muscle fascia avoids the nerve and leads to subperiosteal dissection of the facial skeleton. The supraorbital nerves sometimes restrict ap mobility and dissection of the periorbita. Removal of the bony oor of the foramina using a small osteotome is often required to release the supraorbital neurovascular bundles and permit further forward mobility of the ap. Closure of the incision in layers, even after facial advancement that exceeds 15 mm, is usually not a problem. Before the coronal ap is repositioned, care should be taken to resuspend the lateral canthal tendons. The authors begin by using a small single-prong skin hook to grasp the tendon and lateral canthal soft tissues. Lateral canthopexy is then carried out using a nonresorbable suture material or ne stainless steel suture ligatures placed through the tendon and around the zygoma. Alternatively, a drill hole is made through the lateral orbital rim and the suture is attached. When a pericranial ap is not used, the pericranium may be closed using 4-0 chromic gut sutures. The wound must be irrigated with copious normal saline solution before closure. Once the skin ap is repositioned, closure of the coronal scalp ap is accomplished in layers beginning with interrupted 3-0 vicryl sutures, which are passed through the galea and reapproximate the subcutaneous tissues. Cutaneous closure typically has been accomplished using surgical staples that are maintained for 12 to 14 days. The authors have found the use of absorbable suture materials (chromic gut, vicryl rapide) to be favorable in the closure of coronal aps. This is especially true in pediatric cases in which the use of a resorbable material for closure obviates the need for staple or suture removal during the postoperative period. Oral incisions The use of transoral approaches to the facial skeleton also provides wide exposure while concealing scars, which is an important component of the facial bipartition procedure. Typically, this procedure includes a small midline incision within the maxillary vestibule to create a segmental (sagittal) osteotomy of the maxilla. Although pterygomaxillary disimpaction may be carried out transorally, as performed during a Le Fort I level osteotomy, this requires a larger vestibular incision and area of dissection. The authors preference is to use an osteotome placed from above through the coronal ap for separation of the pterygomaxillary junction.
138
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
The monobloc facial advancement procedure As described previously, the craniofacial skeleton is exposed through a coronal scalp ap approach. Care must be taken to expose the nasal dorsum, internal aspect of the orbits, lateral orbital rims and lateral walls, and zygomatic arch (Fig. 2). After the soft tissue dissection, bifrontal craniotomy is carried out and the frontal bone ap is removed (Fig. 3). The combination of intracranial and subcranial (anterior) approaches allows retraction and protection of the brain and globes so that the procedure may be carried out safely under direct visualization. A reciprocating saw is used to create a small osteotomy through the zygomatic arch (Fig. 4) and then a series of bone cuts through the lateral orbital wall down to the level of the inferior orbital ssure, temporal bone (Tenon extensions), and anterior cranial base (Figs. 58), [17]. When the osteotomy is near the temporal fossa, adequate dissection of the fossa below the sphenoid wing aords protection to the temporal lobe during the osteotomy procedure. This is especially important in patients with Apert syndrome, in whom the temporal lobe tip may extend forward into the lateral orbital rim area. Extension of the osteotomy cuts through the medial orbital wall and orbital oor is then accomplished with the use of a small osteotome. Care must be taken to avoid the infraorbital neurovascular bundle and the nasolacrimal apparatus. Separation of the nasal septal complex from the base of the skull is performed using a large osteotome directed transcranially from the anterior skull base (in front of the cribriform) to the level of the maxillary crest (Fig. 9). Typically, an oral endotracheal tube is used during the initial portion of the operative procedure. Once the nasal septum is divided, the patient may be converted over to a nasal endotracheal tube. In cases in which nasal intubation is used, meticulous care must be taken to avoid damaging the endotracheal tube during this osteotomy. Next, a long, curved osteotome is used to separate the maxilla from the pterygoid plates. This is approached from above through the coronal ap and a nger is placed over the medial surface
Fig. 2. (A, B) Once the coronal ap is elevated, care is taken to expose the entire craniofacial complex so that the osteotomies may be carried out under direct visualization. This includes exposure of the nasal dorsum, internal walls of the orbits, and zygomatic arch in the subperiosteal plane. The temporalis muscle is elevated from the squamosal portion of the temporal bone,which allows access to the lateral orbital walls and the creation of Tenon extensions. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
139
Fig. 3. Transcranial access is provided through bifrontal craniotomy. Initially, bur holes are placed at the margins of the bone ap and on either side of the sagittal sinus, which allows for separation of the dura before the craniotome is used and the bone ap is elevated by the neurosurgeon. The frontal/superior orbital rim unit and Tenon extensions are outlined with a surgical marker before bur holes are placed. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
Fig. 4. Osteotomy through the midportion of the zygomatic arch is completed with a reciprocating saw. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
of the pterygomaxillary junction to conrm complete separation and appropriate placement of the osteotome (Fig. 10). Once the osteotomies are completed, the bone cuts should be tested with a thin osteotome to identify areas of incomplete separation and prevent unintended fractures. Attempts to mobilize facial bones after incomplete osteotomies may result in fracture disruption of the segments, inadequate advancement, and relapse. These problems most frequently occur during movements at the Le Fort III or frontofacial (monobloc) levels. Inadequate separation of the posterior maxillary walls and perpendicular portion of the palatine bone, which are impossible to visualize completely, contributes to this problem and may result in disruption of the zygomatic portion
140
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
Fig. 5. Orbital bone cuts begin by dividing the lateral orbital wall and continue inferiorly to the level of the inferior orbital ssure. An assistant places a malleable retractor within the orbit and retracts the periorbita. The osteotomy is completed under direct visualization. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
Fig. 6. Superiorly, the lateral orbital osteotomy is extended to join the inferior line of the Tenon extension. During this part of the operative procedure, a retractor is placed into the temporal fossa for protection of the temporal lobe. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
of the orbit. The use of osteotomes and specially designed bone spreaders assists in the completion of pterygomaxillary separation and minimizes the risk of associated fractures (Figs. 8, 10, and 11) [13]. When fractures do occur, complete mobilization still must be accomplished. Repair of the involved segments by plate xation is indicated; when this is appropriately performed it seldom results in a problem. Mobilization of the total midfacial skeletal unit is carried out with Rowe disimpaction forceps used to apply slow, controlled, downward force (Fig. 11). The authors preferred approach is to use a prefabricated occlusal splint and arch bars with wire maxillomandibular xation to stabilize the monobloc segment initially. Once the midface has been mobilized with the desired advancement achieved and the patient placed into maxillomandibular xation, attention may be directed to the application of rigid internal xation.
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
141
Fig. 7. The frontal lobes are gently retracted for access to the anterior cranial base. Osteotomy through the orbital roofs is carried out from above using the reciprocating saw, which is continued in front of the cribriform plate in the midline. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
Fig. 8. A small osteotome is used to complete the osteotomies, which allows the surgeon to test potential areas where there has been incomplete separation and prevents unintended fractures. This is especially critical at pterion where complete division with the saw is dicult because of visualization. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
The large skeletal movements carried out during monobloc facial advancements require careful stabilization for successful outcomes. Inadequate stabilization contributes to relapse and infection, and the use of rigid xation minimizes these problems. The application of bone plates and screws for internal xation of the osteotomized segments is done beginning at the zygomatic arches and lateral Tenon extensions. The authors preference is to use resorbable internal xation
142
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
Fig. 9. A larger osteotome is then placed through the cranial fossa osteotomy in front of the cribriform and driven inferiorly to the level of the maxillary crest. The nasal septum is separated from the skull base. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
Fig. 10. Division at the pterygomaxillary junction is accomplished using a longer curved osteotome placed from above through the coronal ap. A nger is placed over the medial aspect of the pterygomaxillary junction to conrm placement of the osteotome and complete separation of the maxilla. Once disimpaction has been carried out, Posnick spreaders are used to mobilize the maxilla adequately. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
wherever possible. The recent evolution in resorbable (polylactic acid polymer) rigid xation systems has made this an attractive option for stabilization of the craniomaxillofacial skeleton. When there is any question that the resorbable xation will withstand the soft tissue relapse forces encountered during midfacial advancements, titanium hardware is used for internal xation. The use of fresh autogenous bone grafts to ll osteotomy gaps provides the most predictable results during orbital and midfacial procedures. Bone grafts are placed into the osteotomy gaps
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
143
Fig. 11. Disimpaction of the total midfacial unit is performed using Rowe disimpaction forceps to apply controlled, downward, and forward traction. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
at the zygomatic arch, along the posterior margin of the advanced frontal bones, and to reconstruct the orbital oors after a monobloc advancement. These grafts are useful in restoring anatomic contours and contribute to the stability of the advanced segments. As a general rule, full-thickness bony defects of the cranial vault should be grafted to ensure adequate regeneration and continuity. This is especially true in children older than 2 years of age, in whom full-thickness skull defects of more than a few millimeters do not heal predictably. The use of split-thickness cranial grafts is favored because of proximity to the surgical site, ease of access through the same coronal ap, and quantities of bone available. The consistency of the bone in the cranium (dense cortical) and its rich haversian network allow it to revascularize quickly and resorb minimally. Calcium phosphate bone cements also may be used for the repair of fullthickness defects and recontouring the cranial vault (Fig. 1G). Modications for facial bipartition The facial bipartition procedure begins with the same surgical steps carried out during a monobloc facial advancement, which are carried out up to and including the complete disimpaction and mobilization of the midface. Before the facial halves may be translocated medially, two additional surgical maneuvers must be carried out. First, midline ostectomy is carried out and a segment of bone from the central face (frontonasal bones) must be removed (Figs. 12 and 1). The specic amount of bone removed varies depending on the individual patients deformity and is predetermined based on presurgical anthropometric and CT-based measurements. Second, the maxilla is segmentalized into two segments using a midline (sagittal) osteotomy (Fig. 13). A nasal-septal osteotome is used to separate the cartilaginous and bony septum from the maxillary crest. The maxilla is then divided in the sagittal plane using a small midline osteotomy created with a bur and then an osteotome for completion. In addition to midfacial advancement, the division of the facial skeleton into two halves permits translocation of the orbits medially for the correction of hypertelorism and forward movement of the lateral orbital rims to correct the arch of the facial form (Fig. 14). The extents to which these movements may be carried out are limited primarily by the palatal soft tissues (Fig. 15). As the facial halves are translocated medially, the most notable change below the level of the orbits is a widening of the maxillary arch form. The forward advancement of the lateral
144
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
Fig. 12. Removal of a fragment of midline bone (frontal and nasal) during facial bipartition. It is often necessary to trim away any residual portions of ethmoidal bone and cartilaginous nasal septum that may interfere with translocation of the facial halves. Care must be taken to minimize tearing of the nasal mucosa. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
orbital rims produces dierential widening, which aects the posterior maxilla. In patients undergoing facial bipartition who have preexisting constriction of the maxillary arch, as is seen in the craniofacial dysostosis syndromes, the surgeon encounters substantial resistance during these skeletal movements. This is especially the case in children with a history of cleft palate repair, in whom palatal scarring results in even greater maxillary transverse collapse and soft tissue immobility.
Fig. 13. Sagittal osteotomy of the maxilla as used in the bipartition procedure. (A) Small midline vestibular incision with exposure of the piriform rim, nasal oor, and septum. (B) Nasal-septal osteotome used for separation of the septum along the maxillary crest. (C and D) A straight osteotome is then used to complete the segmentalization, and small bone spreaders conrm mobility of the two maxillary halves. (From Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995; with permission.)
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
145
Once the orbital medialization is complete, rigid internal xation is applied across the midline. The central region is xated before any bone plates are applied to the lateral Tenon extensions or zygomatic arches. Management of dead space The elimination of dead space during closure of the craniomaxillofacial region is critical for sound surgical practice. Dead space that results from craniofacial procedures is resolved by meticulous closure of tissues, placement of bone grafts, and obliteration with soft tissue aps or free fat. Forward advancements of the craniofacial skeleton during monobloc and bipartition procedures result in the creation of extradural, retrofrontal dead space and communication with the nasal cavity [11]. Potential complications of residual dead space include delayed healing, cerebrospinal uid leaks, and infection. The management of this space in the anterior cranium after frontofacial advancement remains controversial. Expansion of the frontal bones and relatively rapid lling of the residual intracranial space has been well demonstrated in infants and young children. This observation supports the conservative management of dead space in younger patients. More gradual, and less complete, lling occurs in the adult, which may be particularly troublesome when the space communicates directly with the nasal cavity. Sealing the nasal cavity from the cranial fossa is accomplished with primary repair of the nasal mucosa. Because this is not usually feasible, the authors prefer to use an anteriorly based pericranial ap inserted for coverage of the anterior cranial base. The use of brin glue in the recon-
Fig. 14. Skeletal movements before (A) and after (B) facial bipartition. Notice that medial translocation of the upper facial halves to correct orbital hypertelorism also results in some degree of lateral movement involving the maxillary segments.
146
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
Fig. 14 (continued)
struction of the anterior cranial oor also provides a temporary seal between the cavities and allows for re-epithelialization of the nasal mucosa. When forehead procedures are performed and the frontal sinuses are present, management of the dead space is achieved by cranialization, complete removal of the mucosal lining, and obliteration of the nasofrontal ducts with bone grafts or free fat. The placement of bone grafts into bony defects is important for closure of dead space and facilitates rapid healing. These bone grafts should be wedged or stabilized with screws to prevent migration. Defects within the temporal fossa after facial advancements also may be covered nicely with advancement of the temporalis muscles. This procedure eliminates the dead space, and the defect is conned to the hair-bearing area of the scalp. A layered closure of the coronal incision is required for elimination of dead space and an optimal esthetic result. The lateral canthus is stripped during the exposure of the orbital rims, and these structures must be resuspended. Sutures are passed through the canthus and secured to the lateral orbital rim or temporalis muscle fascia. When the temporalis muscle is stripped from the lateral temporal crest or fossa, it should be reattached to the lateral orbital wall and temporal ridge to prevent bitemporal defects. Closure of the subcutaneous tissues and galea is accomplished as a separate layer. Until the nasopharyngeal mucosa seals, communications with the nasal cavity allow air leaks that may result in subcutaneous emphysema or a pneumocephalus. To prevent this type of airow, postoperative endotracheal intubation may be extended or bilateral nasopharyngeal airways placed for a 3- to 5-day period. Sinus precautions and restriction of nose blowing also further limit reux of air and uid during the postoperative period.
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
147
Fig. 15. (A) High arched palate and narrowed maxillary arch width are common ndings in children with craniofacial dysostosis syndromes and craniofrontonasal dysplasia. (B) During the facial bipartition, upper facial movements toward the midline result in lateral expansion of the maxillary arch form. Resistance is encountered from palatal soft tissues at this location.
References
[1] Converse JM, Kazanjian VH. Surgical treatment of facial injuries. Baltimore: Williams and Wilkins; 1949. [2] Tessier P. Osteotomies totales de la face. Syndrome de crouzon, syndrome dapert: oxycephalies, scaphocephalies, turricephalies. Chir Plast Esthet 1967;12(4):27386. [3] Tessier P. Traitement des dysmorphies facials propres aux dysostoses craniofaciales, maladies de crouzon et dapert. Neurochirurgie 1971;17:295322.
148
R.L. Ruiz et al. / Atlas Oral Maxillofacial Surg Clin N Am 10 (2002) 131148
[4] Tessier P. Relationship of craniostenoses to craniofacial dysostosis and to faciostenosis: a study with therapeutic implications. Plast Reconstr Surg 1971;48:22437. [5] Ortiz-Monasterio F, Fuente Del Campo A, Limon-Brown F. Mechanism and correction of V syndrome in craniofacial dysostosis. In: Tessier P, editor. Symposium on plastic surgery in the orbital region. St. Louis: CV Mosby; 1976. p. 24654. [6] van der Meulen JC. Medial faciotomy. Br J Plast Surg 1979;32:339. [7] Tessier P. The monobloc and frontofacial advancement: do the pluses outweigh the minuses? Plast Reconstr Surg 1993;91:988. [8] Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995. [9] Posnick JC. The monobloc and facial bipartition osteotomies: a step-by-step description of the surgical technique. In: Posnick JC, editor. Craniofacial and maxillofacial surgery in children and young adults. Philadelphia: W.B. Saunders; 2000. [10] Posnick JC, Al-Qattan MM, Armstrong D. Monobloc and facial bipartition osteotomies: quantitative assessment of presenting deformity and surgical results based on computed tomography scans. J Oral Maxillofac Surg 1995;53:358. [11] Posnick JC, Al-Qattan MM, Armstrong D. Monobloc and facial bipartition osteotomies for reconstruction of craniofacial malformations: a study of extradural dead space. Plast Reconstr Surg 1996;97:1118. [12] Posnick JC, Nakano P, Taylor M. A modied occlusal splint to avoid tracheotomy for total midface osteotomies. Ann Plast Surg 1992;29:223. [13] Posnick JC, Goldstein JA, Clokie C. Renements in pterygomaxillary dissociation for total midface osteotomies: instrumentation, technique, and CT scan analysis. Plast Reconstr Surg 1993;91:167. [14] Turvey TA, Ruiz RL. Craniosynostosis and craniofacial dysostosis. In: Fonseca RJ, editor. Oral and maxillofacial surgery. Philadelphia: W.B. Saunders; 2001. [15] Posnick JC, Ruiz RL. The craniofacial dysostosis syndromes: a staged reconstructive approach. Cleft Palate Craniofac J 2000;37(5):43357. [16] Waitzman AA, Posnick JC, Armstrong D, et al. Craniofacial skeletal measurements based on computed tomography, Part 2. Normal values and growth trends. Cleft Palate Craniofac J 1992;29:118. [17] Posnick JC. Craniofacial dysostosis syndromes: a staged reconstructive approach. In: Turvey TA, Vig KWL, Fonseca RJ, editors. Facial clefts and craniosynostosis: principles and management. Philadelphia: W.B. Saunders; 1995.
You might also like
- Reconstructive: Plastic and Surgery MDocument4 pagesReconstructive: Plastic and Surgery MashajangamNo ratings yet
- Double Barrel Fibula FlapDocument9 pagesDouble Barrel Fibula FlapAlvaro RivCalleNo ratings yet
- Monobloc and Facial Bipartition Osteotomies For The Reconstruction of Craniosynostosis SyndromesDocument36 pagesMonobloc and Facial Bipartition Osteotomies For The Reconstruction of Craniosynostosis SyndromesCarlos Ccanto ToribioNo ratings yet
- Gomez Et Al 2012Document12 pagesGomez Et Al 2012LAURA MARCELA BARRENECHE CALLENo ratings yet
- Chim 2010Document7 pagesChim 2010nikitagustiNo ratings yet
- Australian Dental Journal: Craniofacial DisordersDocument11 pagesAustralian Dental Journal: Craniofacial DisordersMantili TasrifNo ratings yet
- EDJ - Volume 68 - Issue 1 - Pages 273-280Document8 pagesEDJ - Volume 68 - Issue 1 - Pages 273-280Wilson WijayaNo ratings yet
- Cranial Strains and MalocclusionDocument5 pagesCranial Strains and MalocclusionRyan Dobbeck100% (1)
- Counter Autografting of Dorsal SeptumDocument9 pagesCounter Autografting of Dorsal SeptumÇağlayan YağmurNo ratings yet
- Mandibular Surgery Technologic and Technical Improvements - 2014 - Oral and Maxillofacial Surgery Clinics of North AmericaDocument35 pagesMandibular Surgery Technologic and Technical Improvements - 2014 - Oral and Maxillofacial Surgery Clinics of North AmericaGabriela Lizbeth ArmentaNo ratings yet
- Panfacial TraumaDocument104 pagesPanfacial TraumaMehek BatraNo ratings yet
- Wolford 1994 - Occlusal Plane Alteration in Orthognathic Surgery, EstheticsDocument13 pagesWolford 1994 - Occlusal Plane Alteration in Orthognathic Surgery, EstheticsBillNo ratings yet
- Corticotomia y Canteamiento Plano OclusalDocument11 pagesCorticotomia y Canteamiento Plano OclusalKarla SolísNo ratings yet
- Primary RhinoplastyDocument7 pagesPrimary Rhinoplastysmansa123No ratings yet
- Lin 2021Document14 pagesLin 2021fabian hernandez medinaNo ratings yet
- Onlay Bone Graft Augmentation For Refined Correction of Coronal SynostosisDocument9 pagesOnlay Bone Graft Augmentation For Refined Correction of Coronal Synostosisbalab2311No ratings yet
- Correction of Late Adolescent Skeletal Class III Using The Alt-RAMEC Protocol and Skeletal AnchorageDocument11 pagesCorrection of Late Adolescent Skeletal Class III Using The Alt-RAMEC Protocol and Skeletal AnchoragePorchiddioNo ratings yet
- 10.2 Major Surgical ProceduresDocument22 pages10.2 Major Surgical ProceduresishtiiiNo ratings yet
- Navigation-Guided Reduction and Orbital Floor Reconstruction in The Treatment of Zygomatic-Orbital-Maxillary Complex FracturesDocument7 pagesNavigation-Guided Reduction and Orbital Floor Reconstruction in The Treatment of Zygomatic-Orbital-Maxillary Complex FracturesBriando Stevano LinelejanNo ratings yet
- Maxillary Arch Distalization Using Interradicular Miniscrews and The Lever-Arm ApplianceDocument8 pagesMaxillary Arch Distalization Using Interradicular Miniscrews and The Lever-Arm ApplianceJuan Carlos CárcamoNo ratings yet
- Apert N CrouzonDocument13 pagesApert N CrouzonfayasyabminNo ratings yet
- 10 1016@j Ijom 2013 07 541Document1 page10 1016@j Ijom 2013 07 541leslie kalathilNo ratings yet
- InTech-Basic and Advanced Operative Techniques in Orthognathic SurgeryDocument22 pagesInTech-Basic and Advanced Operative Techniques in Orthognathic Surgerybayyin nurrahmiNo ratings yet
- Pos Nick 2004Document16 pagesPos Nick 2004Bhárbara Marinho BarcellosNo ratings yet
- Orthognathic Surgery & Lefort 1 OsteotomiesDocument71 pagesOrthognathic Surgery & Lefort 1 OsteotomiesNikhilAsokNo ratings yet
- Fronto-Orbital Advancement Using An en Bloc Frontal Bone CraniectomyDocument7 pagesFronto-Orbital Advancement Using An en Bloc Frontal Bone Craniectomyandredwijaya8No ratings yet
- Asimetrii MandibulareDocument25 pagesAsimetrii MandibulareBranici OanaNo ratings yet
- 4 Suturas RinoplastiaDocument5 pages4 Suturas RinoplastianefimdNo ratings yet
- Xu 2014Document10 pagesXu 2014Marcos Gómez SosaNo ratings yet
- 1 s2.0 S2666430522000036 MainDocument12 pages1 s2.0 S2666430522000036 MainDANTE DELEGUERYNo ratings yet
- Fu 2016Document10 pagesFu 2016Soe San KyawNo ratings yet
- Manual Ao Cirurgia OrtognáticaDocument35 pagesManual Ao Cirurgia OrtognáticaAndre Luis CostaNo ratings yet
- Kim (2021)Document8 pagesKim (2021)Caio GonçalvesNo ratings yet
- Case Report Treatment of An Adult Skeletal Class III Patient With Surgically Assisted Rapid Palatal Expansion and FacemaskDocument7 pagesCase Report Treatment of An Adult Skeletal Class III Patient With Surgically Assisted Rapid Palatal Expansion and FacemaskMirza GlusacNo ratings yet
- Park 2012Document6 pagesPark 2012Yassin SalahNo ratings yet
- Midfacial Reconstruction Using Calvarial Split Bone GraftsDocument6 pagesMidfacial Reconstruction Using Calvarial Split Bone Graftsbalab2311No ratings yet
- CirugiaDocument27 pagesCirugiaalexmtzgNo ratings yet
- Aesthetic Considerations in Orthofacial SurgeryDocument10 pagesAesthetic Considerations in Orthofacial SurgeryCristian MartinezNo ratings yet
- A Classification System of DefectsDocument12 pagesA Classification System of DefectsPrana SkyaNo ratings yet
- Classification of Angle Class III MalocclusionDocument11 pagesClassification of Angle Class III MalocclusionAnda TarasciucNo ratings yet
- Articulo ProtodonciaDocument5 pagesArticulo ProtodonciaMilton David Rios SerratoNo ratings yet
- The Osseous Genioplasty: Clinicsin Plastic SurgeryDocument16 pagesThe Osseous Genioplasty: Clinicsin Plastic SurgeryRajan KarmakarNo ratings yet
- FHKTHKGDocument6 pagesFHKTHKGRehana SultanaNo ratings yet
- 2023 Crooked NosesDocument9 pages2023 Crooked NosesJuliann PedroNo ratings yet
- Comlicaciones Osteotomia Saguital de RamaDocument4 pagesComlicaciones Osteotomia Saguital de RamaLeonardCarreraDiazNo ratings yet
- Free Flaps MaxillaDocument7 pagesFree Flaps MaxillaFahad QiamNo ratings yet
- A Countdown To Orthognathic SurgeryDocument5 pagesA Countdown To Orthognathic SurgeryhaneefmdfNo ratings yet
- Secondary Deofrmities of Cleft Lip NoseDocument8 pagesSecondary Deofrmities of Cleft Lip NoseahmedatefNo ratings yet
- 10.2478 - Aoj 1980 0007Document10 pages10.2478 - Aoj 1980 0007Naliana LupascuNo ratings yet
- Orthodontic Aspects of Orthognathic Surgery - Shiva ShankarDocument57 pagesOrthodontic Aspects of Orthognathic Surgery - Shiva Shankarnevin santhosh100% (1)
- Litrature Study: Reinforced Devices Are Superior in Terms of Mechanical Strength and Handling PropertiesDocument3 pagesLitrature Study: Reinforced Devices Are Superior in Terms of Mechanical Strength and Handling PropertieshggjNo ratings yet
- TREATMENT Algorithm For Bilateral Alveolarcleft Based On The Position of The Premaxillaand The Width of The Alveolar GapDocument7 pagesTREATMENT Algorithm For Bilateral Alveolarcleft Based On The Position of The Premaxillaand The Width of The Alveolar GapFranklin HaroNo ratings yet
- Chin ImplantDocument6 pagesChin ImplantAnkita GuravNo ratings yet
- Non-Vascularized Auto Bone Grafting in Segmental Defect of Mandible in Treatment of Benign TumorDocument5 pagesNon-Vascularized Auto Bone Grafting in Segmental Defect of Mandible in Treatment of Benign TumorRajan KarmakarNo ratings yet
- 2010 Miniscrew Assisted Nonsurgical PalatalDocument10 pages2010 Miniscrew Assisted Nonsurgical PalatalMariana SantosNo ratings yet
- Journal of Cranio-Maxillo-Facial Surgery: Case ReportDocument6 pagesJournal of Cranio-Maxillo-Facial Surgery: Case Reportnh7p6yctv8No ratings yet
- Openbite MIA AJODODocument10 pagesOpenbite MIA AJODOscribdlptNo ratings yet
- Jawline Contouring AlfaroDocument5 pagesJawline Contouring AlfaroÂngelo Rosso LlantadaNo ratings yet
- Disorders of the Patellofemoral Joint: Diagnosis and ManagementFrom EverandDisorders of the Patellofemoral Joint: Diagnosis and ManagementNo ratings yet
- Design and Fabrication of A Blanking Tool: Gopi Krishnan. C (30408114309) (30408114092)Document44 pagesDesign and Fabrication of A Blanking Tool: Gopi Krishnan. C (30408114309) (30408114092)Daniel Saldaña ANo ratings yet
- April 5, 2018 Engr Alex Relampagos Subject: Supply, Delivery, Installation of Fire Detection and Alarm SystemDocument2 pagesApril 5, 2018 Engr Alex Relampagos Subject: Supply, Delivery, Installation of Fire Detection and Alarm SystemAlexander Luega Relampagos0% (1)
- Internet Programming With Delphi (Marco Cantu)Document14 pagesInternet Programming With Delphi (Marco Cantu)nadutNo ratings yet
- Demographic Indicators133750362Document26 pagesDemographic Indicators133750362Longyapon Sheena StephanieNo ratings yet
- Pole W Solar Panel - Design ReportDocument62 pagesPole W Solar Panel - Design ReportGaurav Sharma100% (4)
- Buckling and Ultimate Strength Assessment For Offshore Structures APRIL 2004Document5 pagesBuckling and Ultimate Strength Assessment For Offshore Structures APRIL 2004Flávio RodriguesNo ratings yet
- Infant BathingDocument2 pagesInfant BathingAira SantosNo ratings yet
- GH2023 - 5876 Saturin & Minh Enhancing Efficiency of Geotechnical Design For Offshore MonopileDocument7 pagesGH2023 - 5876 Saturin & Minh Enhancing Efficiency of Geotechnical Design For Offshore MonopileMinh Nguyen-AnhNo ratings yet
- IT0007-Laboratory-Exercise-7 - Incident HandlingDocument5 pagesIT0007-Laboratory-Exercise-7 - Incident HandlingDenise JaoNo ratings yet
- Acid Jurnal 4Document7 pagesAcid Jurnal 4awaloeiacidNo ratings yet
- Barium StudiesDocument89 pagesBarium StudiesManuel PoncianNo ratings yet
- Brainaid CaseDocument4 pagesBrainaid CaseJyotiraditya Kumar JhaNo ratings yet
- Necromancer Bone Spear Build With Masquerade (Patch 2.6.10 Season 22) - Diablo 3 - Icy Veins 3Document1 pageNecromancer Bone Spear Build With Masquerade (Patch 2.6.10 Season 22) - Diablo 3 - Icy Veins 3filipNo ratings yet
- Village Cleanliness Index & SLWM IndexDocument16 pagesVillage Cleanliness Index & SLWM Indexpunu904632No ratings yet
- 3.2.4 - Design Info-Water SupplyDocument5 pages3.2.4 - Design Info-Water SupplyNyu123456No ratings yet
- FIN515 Hw4JDVDocument7 pagesFIN515 Hw4JDVvalderramadavid100% (1)
- Pega74 Install Tomcat Db2Document43 pagesPega74 Install Tomcat Db2Amit GujralNo ratings yet
- Smiths of Winterforge Rulebook 0.9Document9 pagesSmiths of Winterforge Rulebook 0.9NatsukiPLNo ratings yet
- Mcdonald's - Group 5Document57 pagesMcdonald's - Group 5Thanh Trà100% (1)
- Felcom 15 Servi̇s ManualDocument236 pagesFelcom 15 Servi̇s ManualAnıl Kahya75% (8)
- Financial Institution Failure Prediction Using Adaptive Neuro-Fuzzy Inference Systems: Evidence From The East Asian Economic CrisisDocument2 pagesFinancial Institution Failure Prediction Using Adaptive Neuro-Fuzzy Inference Systems: Evidence From The East Asian Economic CrisisHannan KüçükNo ratings yet
- TSX Cusb485Document2 pagesTSX Cusb485AurellioNo ratings yet
- Emerging Trends in Sales ManagementDocument14 pagesEmerging Trends in Sales ManagementAbdul Quadir100% (7)
- Analysis of Portal FrameDocument16 pagesAnalysis of Portal FrameKanchana RandallNo ratings yet
- Overview of EU Funds For Research and InnovationDocument8 pagesOverview of EU Funds For Research and InnovationFacultad de Ingeniería U de ANo ratings yet
- LK-1910, LK-1920, LK-1930 emDocument187 pagesLK-1910, LK-1920, LK-1930 emAmila LasanthaNo ratings yet
- Ohio Department of Transportation: Highway Plan Reading ManualDocument68 pagesOhio Department of Transportation: Highway Plan Reading ManualMwesigwa Dani100% (1)
- 123Document10 pages123Aryan SanezNo ratings yet
- GM - January 3-5, 2024Document3 pagesGM - January 3-5, 2024jNo ratings yet
- Red Hat System Administration I 3.4 PracticeDocument9 pagesRed Hat System Administration I 3.4 PracticestefygrosuNo ratings yet