Professional Documents
Culture Documents
4
4
Uploaded by
amirswtCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
4
4
Uploaded by
amirswtCopyright:
Available Formats
Thermochimica Acta 453 (2007) 113119
Light mirror reection combined with heating/cooling curves as a method of studying phase transitions in transparent and opaque petroleum products: Apparatus and theory
Yu.L. Shishkin
Gubkin Russian State University of Oil and Gas, Leninsky Prospect 65, 119991 Moscow, Russia Received 6 March 2006; received in revised form 31 October 2006; accepted 12 November 2006 Available online 21 November 2006
Abstract A portable low weight low cost apparatus Phasafot and method for determining pour and cloud points of petroleum products, as well as precipitation and melting temperatures of parafns in both transparent (diesel fuels), semi-transparent (lube oils) and opaque (crude oils) samples are described. The method consists in illuminating the surface of a sample with an oblique light beam and registering the intensity of specularly reected light while heating/cooling the sample in the temperature range of its structural transitions. The mirror reection of a light beam from an ideally smooth liquid surface falls in intensity when the surface becomes rough (dim) due to crystal formation. Simultaneous recording of the temperature ramp curve and the mirror reection curve enables the determination of the beginning and end of crystallization of parafns in both transparent and opaque petroleum products. Besides, their rheological properties can be accurately determined by rocking or tilting the instrument while monitoring the sample movement via its mirror reection. 2006 Elsevier B.V. All rights reserved.
Keywords: Light mirror reection; Petroleum products; Parafns precipitation; Pour point; Cloud point; Heating/cooling curves
1. Introduction The propensity of petroleum products to precipitate parafns may cause the obstruction of crude oil pipe-line ow, plugging of lters in motor engines and in rening operations, gelation of fuels in car engines, etc. Therefore, the cloud point the temperature at which the parafn crystal formation becomes visible in a fuel, and the pour point the temperature at which the product loses its mobility are important technological parameters for whose determination a number of instruments have been devised and marketed. Although these instruments serve their purpose in most cases, they have a number of shortcomings, among which is their inability to measure crystallization and melting temperatures of parafns in opaque samples, e.g. crude oils and their heavy residues. Besides, the measurement of pour points of various petroleum products is often difcult to perform with sufcient precision, or the instrumentation is cumbersome and expensive. Therefore, attempts are constantly
E-mail address: yu.l.shishkin@mail.ru. 0040-6031/$ see front matter 2006 Elsevier B.V. All rights reserved. doi:10.1016/j.tca.2006.11.010
being made to develop new or upgrade the existing instruments. Methods based on DSC [1], thermal microscopy, viscometry, and DSC [24], DSC and viscometry [5] may be mentioned in this connection, as they permit a detailed and objective study of petroleum products by comparatively simple means. A wide range of tasks can be solved including the improvement of pour point depressant formulations and the study of the mechanism of pour point depression [4]. An added advantage of thermal microscopy is its ability to study opaque samples, e.g. the processes of parafn crystal growth in crude oils [2,3]. Still, the instruments employed are expensive, and the analysis takes time. Wax (parafn) deposition is an ever present problem in pipelines and oil wells. In Ref. [6] application of CT imaging has been proposed as an alternative tool for cloud point determination. In Refs. [7,8] differential scanning calorimetry has been used to establish the wax content and wax appearance temperatures of crude oils. In Ref. [9] the limitations of the cloud point measurement techniques and the inuence of the oil composition on its detection are discussed. Theoretical aspects of the problem of wax deposition in wells and pipelines can be found in Refs. [10,11].
114
Yu.L. Shishkin / Thermochimica Acta 453 (2007) 113119
In the present communication a simple and rapid method to study the low temperature properties of various crude oils and related products is described. 2. Results and discussion A method alternative to those used currently in the petroleum industry is presented hereinafter in some detail. It will be shown in what follows that the shortcomings of the known methods can be overcome by adopting a new technique of photometric signal generation and measurement. This consists in registering the light reected from the surface of a liquid sample undergoing various phase transitions during heating or cooling. In the liquid state the opaque sample has an ideally smooth surface capable of specularly reecting about 45% of the light falling on it for the incidence angle of about 30 . As soon as a phase transition, e.g. crystallization sets in, the crystals appearing in the bulk and hence on the surface of the sample make the surface rough due to chaotic orientation of the surface crystals. As a result the randomly scattered portion of the light increases, while the specularly reected portion diminishes in proportion to the crystal density on the surface. Monitoring the intensity of the specularly reected or randomly scattered light rather than that of the light transmitted (as in common methods) enables the study of phase transitions in samples of various nature, texture, and transparency. The situation described above applies mainly to opaque or semi-opaque samples. In the latter case the use of cuvettes with blackened bottoms is recommended to reduce the light reected from the bottom and leave only that reected from the surface. In the case of transparent and semi-transparent samples a different mode of operation is adopted. A liquid sample forming a 25 mm thick layer is placed in a metal cuvette with a at polished bottom. The light is reected back from the cuvette bottom to the photoreceptor, passing twice the sample, which enables operation in the conventional transmission mode in which the optical densities of liquid samples can be studied. In this case the specular component from the surface should be minimized as it serves no useful purpose, and that from the bottom maximized. The borderline between the two modes of operation is determined by the optical properties of the sample, viz. its optical density. A schematic drawing of the apparatus Phasafot is presented in Fig. 1. It consists of the following basic parts: outer jacket 1 and inner jacket 2 covered with lid 3: optical unit 4 housing reectometer and temperature sensor (thermocouple); heating/cooling device based on Peltier elements 5 and 6 connected to temperature programmer 7. Inlet 8 and outlet 9 are provided in the jacket for passing the water cooling the Peltier elements. A cup 10 with sample is put on the Peltier device and the optical unit 4 lowered into the thermostat. Simultaneously the thermocouple 11 protruding from the optical unit is immersed in the sample. A light emitting device LED 13 projects a beam of light onto the surface of the sample at an angle about 30 relative to the perpendicular to the surface plane; the beam of light is
Fig. 1. Schematic drawing of apparatus Phasafot.
reected back at the same angle and falls on the photoreceptor 12 which generates a signal (voltage) proportional to the intensity of the reected beam. The analogue signals (voltages) from the reectometer and the thermocouple are fed into the ADB 14 where they are digitized and then passed on to the computer 15. The digitized signals are collected by the program Thermo and displayed on the monitor screen as two curvesthe sample mirror reection (MR) and the sample temperature (T) as functions of time (Fig. 2). Heating/cooling rates may vary from 2 to 20 C/min, the temperature range of the instrument is from 50 to 120 C. When determining crystallization temperatures of parafns in petroleum products and their pour points the mode of the instrument operation is the following. The cup is lled with 0.40.5 ml of the liquid under study and placed in the thermostat, the optical unit placed above the cup and the instrument positioned horizontally to obtain a maximal MR signal. The sample is heated to 4050 C to eliminate its thermal history, then subjected rst to a cooling and then to a heating cycle. The temperatures of phase transitions are noted visually using the changes in the MR signal in the course of the experiment or by recording the curves via the computer. Below are given examples of the instrument operation. 2.1. Determination of crystallization/melting temperatures of samples transparent in the liquid state (e.g. individual parafns) In this example the sample is transparent in the liquid state, thus a cuvette with light reecting bottom is used. The solid parafn is loaded into the cup in the amount sufcient to form a lm 23 mm thick completely covering the cup bottom on melting. On Fig. 2 mirror reection (MR) and heating/cooling (H/C) curves are presented as they are seen on the monitor screen after the run. Time is plotted on the X-axis and the sample temperature on the ordinate. The MR curve is dimensionless. The temperature curve is composed of three cycles: heating, cooling, and then again heating. A horizontal step is
Yu.L. Shishkin / Thermochimica Acta 453 (2007) 113119
115
Fig. 2. Curve 1: mirror reection (dimensionless), curve 2: temperature of nonadecane C19 H40 (plotted on the Y-axis). The upward step on the MR curve indicates parafn melting in the heating cycle, the downward step indicate freezing of the melt in the cooling cycle.
observed on the temperature curve and an upward step on the MR curve on the parafn melting at 30 C in the rst heating cycle, see the left side of Fig. 2. On melting the solid parafn forms a transparent lm on the cup bottom, and the increased light reected from the cuvette causes the rise of the MR signal. In the cooling cycle the liquid parafn begins to solidify at 30 C and a horizontal step appears on the cooling curve. The downward step on the MR curve occurs just at the beginning of the horizontal step on the cooling curve. This is due to a rapid and uniform fall-out of parafn crystal nuclei which make the sample opaque thus reducing the mirror reection from the cuvette bottom. The further growth of the crystals within the duration of the horizontal step does not change the MR to any great extent. In the second heating cycle (see the right side of Fig. 2) as the sample melts and becomes transparent again the MR signal rises causing an upward step on the MR curve. This step is found at the end of the quasi-horizontal (sloped) step on the heating curve. This is due to the opaque parafn lm persisting on the sample surface till its full melting.
A reversible rotatory phase transition can also be discerned on the cooling and heating curves marked by small sloped steps on the C/H curves at around 20 C and corresponding upward/downward steps on the MR curve, due evidently to a better reection ability of the parafn non-rotatory phase formed below 20 C on cooling. In the above example the cooling/heating curves alone may be used for studying the parafn crystal formation/melting due to the large heats absorbed/evolved at nearly constant temperature producing horizontal steps on H/C curves. In the case of mixtures (technical parafns) or low purity specimens with broadened transition intervals or solutions with moderate parafn content no well dened steps are observed on the temperature curve making them unusable for establishing transition temperatures. The MR curves of a technical parafn are given below as a typical example (Fig. 3). Although no steps can be discerned on the temperature curve there are well dened steps on the MR curve enabling the exact pinpointing of phase transition temperatures in the sample (if used together with the temperature curve). The irregular doublepeak shape of the MR curve in the melting region of the second
Fig. 3. Curve 1: mirror reection, curve 2: sample temperature of a technical parafn (presumably a C22 H46 C28 H58 mixture).
116
Yu.L. Shishkin / Thermochimica Acta 453 (2007) 113119
Fig. 4. MR cooling curve 1 and MR heating curve 2 of Kumkol crude oil.
heating cycle points to the presence in the sample of low and high-melting parafn fractions with Tm = 68 C and Tm = 79 C, respectively. 2.2. Determination of freezing/melting temperatures of parafns in opaque solutions (e.g. crude oils) In the case of opaque samples no light is reected from the cuvette bottom and only that reected from the sample surface comes into play. Although its intensity is small (45% of the total incident light) as compared to that reected from the metal cuvette bottom (up to 99%), it can produce a high voltage signal (in our case 56 V) quite sufcient for very sensitive measurements. The gure shows that the freezing and melting of parafns in the crude can be very well seen due to the steep downward and upward steps on the MR curve in the region of crystal formation/melting (precipitation/dissolution), while not a trace of these events can be discerned on the ideally uniform C/H curves. Projecting the steps of the MR curve on the C/H curves allows pinpointing the temperatures of phase transitions in the sample. Data of Fig. 4 was processed to obtain a graph giving the percent content D of the solid fraction as a function of the melt temperature (Fig. 5). It was calculated using the formula D = 100 h % H
The parafns in the crude precipitate in the interval 10 to (15) C and melt in the interval 20 to (+16) C, their being a negative hysteresis of 11 C between the freezing and melting curves at 80% conversion. 2.3. Determination of pour points of crude oils The mirror reection can also be used for studying the mechanistic (rheological) properties of petroleum products, viz. their ability to ow or solidify on heating/cooling. For this purpose the instrument is tilted periodically in the temperature range where the sample undergoes the process of solidication. For the liquid sample this results in a rough comb-like appearance of the MR curve due to the periodic violation of the parallel disposition of the planes of the liquid sample surface and that of the photoreceptor (the MR signal is maximal when these planes are parallel). On the sample becoming solid the MR curve becomes at due to the said planes becoming xed at some constant angle. The point on the cooling curve where the MR becomes constant despite the instrument rocking is the temperature Tg of the sample solidication (gelation), see Fig. 6. After this the pour point of the sample can be determined in the heating cycle. If the sample-photoreceptor planes happened
where h is the current height of the downward step on the MR curve and H is the full height of the step. The left curve on the graph refers to the cooling cycle; it is obtained on moving from high to low temperatures (from the right to the left on the gure), and the right curve refers to the heating cycle; it is obtained on moving from low to high temperatures, i.e. from left to right on the gure. It is seen on Fig. 4 that parafn precipitation begins just at the start of the downward deection on the curve, occurring at 10 C, which is the top point of the left portion of the curve on Fig. 4 and the right portion of the cooling curve on Fig. 5. The slight rise of MR just before its fall may be due to gel (glass) formation of the crude just preceding parafn precipitation.
Fig. 5. Data of Fig. 4 presented as solid fraction D, %; temperature, C graph. Curve 1: precipitation of parafns, curve 2: their melting.
Yu.L. Shishkin / Thermochimica Acta 453 (2007) 113119
117
Fig. 6. MR cooling curve 1 of Kumkol crude oil obtained on rocking the instrument. Gelation point Tg = 20 C. MR heating curve 2 obtained without rocking. Pour point Tp = 20 C.
to be parallel on solidication causing a maximal MR signal, the instrument is left tilted during heating so that when the sample melts and ows at its pour point the MR signal falls, thus enabling the pour point identication. If, on the contrary, the sample-photoreceptor planes were not parallel when the sample became solid, and its MR was not maximal, the instrument is positioned horizontally during the heating cycle; on reaching the pour point the sample ows, the said planes become parallel and the MR signal returns to maximum, thus enabling the pour point (Tp ) identication. The latter case is exemplied in Fig. 6 where the MR signal of the Kumkol crude oil happened to be at its lowest on the sample becoming solid. From the MR curve one nds the solidication (gelation) temperature of the crude Tg = 20 C and the pour point Tp = 20 C, i.e. the temperatures Tg and Tp are identical (the transition from liquid to solid and from solid to liquid is quite reversible). It should be noted in the present context that the term solidication is not equivalent to the term crystallization. Comparing Figs. 5 and 6 one can see that the Kumkol crude solidies at 20 C, but precipitation (crystallization) of parafns in the crude occurs at much lower temperatures Tcl = 10 to (12) C. Solidication of the crude is in fact gelationthe formation of a non-Newtonian liquid, otherwise immobile but capable of ow if a proper shearing stress is applied to it. The gel,
being a structured liquid, reects light as a liquid and therefore is not accompanied by the fall of MR, in contrast to crystallization. On the contrary, a slight rise of the MR signal can be observed beginning from 17.4 C (Fig. 5), which is close to Tg = 20 C of Fig. 6. Evidently, the gel reects light slightly better than the liquid, and this produces a small upward deection on the MR curve just before parafn precipitation. Thus, from Figs. 5 and 6 it follows that gelation precedes parafn precipitation in the cooled crude oil, and not vice versa, as is commonly thought. From the above it follows that the cloud point of the Kumkol crude is Tcl = 10 C and its gelation and pour points are Tg = Tp = 20 C. 2.4. Determination of cloud and pour points of a diesel fuel In the present example the sample has solidied at a nearly maximum MR signal, and this has made possible monitoring the sample gelation and parafn precipitation in the same run. The fall of MR in the interval 9(8) to (20) C shows that parafn crystals form in this interval just after the sample has become solid. So its gelation Tg = 8 or 9 C and its cloud point Tcl = 8 or 9 C (Fig. 7). After this the instrument was tilted and the heating cycle started. The formed crystals melt in the gel in the interval 18
Fig. 7. MR cooling curve 1 of a summer diesel fuel obtained on rocking the instrument. Gelation point Tg = 8 C. MR heating curve 2 obtained without rocking. Parafns melting interval Tm = 20 to (8) C, pour point Tp = 8 C.
118
Yu.L. Shishkin / Thermochimica Acta 453 (2007) 113119
Fig. 8. MR cooling curve 1 and MR melting curve 2 of industrial base oil I-20A.
to (8) C (the MR rises beginning from 18 C), then the gel melts in its turn and the sample ows causing an abrupt fall of MR at 8 C. So the sample pour point is Tp = 8 C. In this example parafn precipitation begins immediately after gelation without overcooling (in contrast to crude oils) evidently due to the absence in the fuel of resins and asphaltenes depressants of parafn crystallization. Here both the fuel gelation and parafn precipitation are fully reversible and very close to each other (proceed without overcooling). 2.5. Determination of lube base oil pour and cloud points Cloud and pour points of a light industrial base oil were determined by the described method (Figs. 8 and 9). The light base oil fully loses mobility at 28 C; at this temperature the comb on the graph levels off. So its gelation point is Tg = 28 C. On heating the gelled sample (without rocking) the latter melts in the interval 24 to (22) C as is shown by the downward step on the MR heating curve. So its average pour point is Tp = 23 C. Thus, there is a hysteresis of 5 C between the gel formation and gel melting temperatures Tg
and Tp . These may be considered as two pour points of the light base oilone obtained on cooling (Tg ), and the other on heating (Tp ). To study the parafn precipitation and dissolution (melting) in the base oil the instrument was positioned horizontally to obtain a maximum MR signal (and thus maximum sensitivity of measurement), and the cooling cycle started. Fig. 9 shows that the light reecting ability of the base oil begins to rise from 4 C. It begins to fall at 15 C due evidently to the start of parafn precipitation, so its cloud point is Tcl = 15 C. The last fraction of small low melting parafn crystals form in the interval 24 to (28) C, which is close to the base oil gelation point Tg = 28 C (temperature of its full immobilization). In the heating cycle the parafns melt at 24 to (22) C, which is close to the sample pour point Tp = 23 C (the temperature of its liquefaction and ow). In this example two distinct parafn fractions are present in the sample: the high melting one with Tcl = 15 C and the low melting one with Tcl = 24 to (28) C. The precipitation of this fraction, predominant in the sample, coincides with its gelation, just as in the case of the diesel fuel discussed above. The same
Fig. 9. Crystallization (curve 1) and melting (curve 2) of parafns of base oil I-20.
Yu.L. Shishkin / Thermochimica Acta 453 (2007) 113119
119
refers to the temperatures of parafn crystals melting (Tm ) and the sample ow (Tp ). 3. Conclusions 1. The method described above has a number of distinctive features offering the researcher quite a number of new possibilities as regards the study of low temperature properties, thermal as well as rheological, of various petroleum and not only petroleum products. For the rst time mirror reection from liquid opaque samples has been made full use of to obtain important information on events taking place in such nontransparent objects. On the other hand, its use in combination with light reecting cuvettes has made possible work in transmission mode and thus enabled traditional optical density measurements, and its use in combination with mechanical perturbation of the sample has much simplied determination of the rheological properties of materials. 2. Application of the method to petroleum products has revealed the following sequence of events taking place in such products, e.g. crude oils. On cooling: gelation (glass formation) parafn nuclei fall-out (precipitation) growth of nuclei (parafn crystal formation). On heating: melting of parafn crystalsmelting of the gel leading to the sample liquefaction (ow). 3. The notions of pour point and cloud point of petroleum products should be reconsidered and made more meaningful and precise in the light of the obtained results. Actually, there are two pour points: one obtained on cooling (Tg ) and the other
on heating (Tp ). They may or may not coincide depending on the nature of the product, e.g. absence or presence in it of resins and asphaltenes. The same applies to the cloud point. On cooling the cloud point Tcl is the temperature of the nuclei fall-out often occurring with considerable overcooling, and on heating it is the temperature Tm of the melting of the last parafn crystals. These may be quite different temperatures. Besides, the cloud and pour points are not actually points, but intervals, whose breadth is proportional to the molecular and structural heterogeneity of the sample. 4. The universal character of the proposed method, its high precision and high through-put capacity recommend it well for use in laboratories of petroleum product quality assessment and control. References
[1] P. Claudy, J.M. Letoffe, B. Chague, J. Orrit, Fuel 67 (1988) 58. [2] M.V. Kok, J.M. Letoffe, P. Claudy, D. Martin, M. Garcin, J.L. Volle, Fuel 75 (1996) 787. [3] J.M. Letoffe, P. Claudy, M.V. Kok, M. Garcin, J.L. Volle, Fuel 74 (1995) 810. [4] M.V. Kok, J.M. Letoffe, P. Claudy, D. Martin, M. Garcin, J.L. Volle, J. Thermal Anal. 49 (1997) 727. [5] M.V. Kok, J.M. Letoffe, P. Claudy, J. Thermal Anal. Calorimetry 56 (1999) 959. [6] O. Karacan, B. Demiral, M.V. Kok, Petrol. Sci. Technol. 18 (2000) 835. [7] J. Chen, J. Zhang, H. Li, Thermochim. Acta 410 (12) (2004) 2326. [8] Z. Jiang, J.M. Hutchinson, C.T. Imrie, Fuel 80 (2001) 367. [9] J.A.P. Coutinho, J.-L. Daridon, Petrol. Sci. Technol. 23 (2005) 1113. [10] M.V. Kok, O. Saracoglu, Petrol. Sci. Technol. 18 (2000) 1121. [11] E. Ozbayoglu, B. Demiral, M.V. Kok, Petrol. Sci. Technol. 18 (2000) 177.
You might also like
- Filmdropwise LabDocument47 pagesFilmdropwise Labaizaqsyazwan50% (2)
- Film and Drop Condensation.11jan17Document15 pagesFilm and Drop Condensation.11jan17Justin D. BrownNo ratings yet
- Fundamentals ThoughtsDocument21 pagesFundamentals ThoughtsNicole Casanova0% (1)
- Coefficient of ViscosityDocument13 pagesCoefficient of Viscositydualpower1983% (6)
- ME 315 - Heat Transfer Laboratory Experiment No. 5 Pool Boiling in A Saturated LiquidDocument10 pagesME 315 - Heat Transfer Laboratory Experiment No. 5 Pool Boiling in A Saturated LiquidAswith R ShenoyNo ratings yet
- Organic Rankine CycleDocument14 pagesOrganic Rankine Cycleoverlord5555No ratings yet
- ! 19 Organic Rankine CycleDocument14 pages! 19 Organic Rankine Cyclesapcuta16smenNo ratings yet
- Two Phase Heat Transfer - MSTDocument10 pagesTwo Phase Heat Transfer - MSTsukhmaniNo ratings yet
- Interpretation of DSC Curves in Polymer Analysis 2000 - ToledoDocument0 pagesInterpretation of DSC Curves in Polymer Analysis 2000 - ToledoyrecoverNo ratings yet
- Expt 8 DSCDocument6 pagesExpt 8 DSCkalendakbNo ratings yet
- The Austenitic Start and Finish Temperature of A Ni55Ti45 Compression SpringDocument22 pagesThe Austenitic Start and Finish Temperature of A Ni55Ti45 Compression SpringRavi AcharyaNo ratings yet
- Analysis of Heat Transfer During Quenching of A Gear Blank: UCRL-JC-133520 PreprintDocument32 pagesAnalysis of Heat Transfer During Quenching of A Gear Blank: UCRL-JC-133520 PreprinthsemargNo ratings yet
- Differential Scanning CalorimetryDocument6 pagesDifferential Scanning CalorimetrySurender MalikNo ratings yet
- CFD Analysis On Thermal Energy Storage in Phase Change MaterialsDocument9 pagesCFD Analysis On Thermal Energy Storage in Phase Change MaterialsDheeraj MiglaniNo ratings yet
- Com Ac - DRC (2007) CMT 2007 2696 4 - enDocument171 pagesCom Ac - DRC (2007) CMT 2007 2696 4 - enAnonh AdikoNo ratings yet
- Turbine Blade Cooling by Air Using Different Methods: September 2011Document23 pagesTurbine Blade Cooling by Air Using Different Methods: September 2011Badr BouananeNo ratings yet
- AT 0 Lab Report PDFDocument7 pagesAT 0 Lab Report PDFerlanggasulaiman90No ratings yet
- The CFD Simulation of Temperature Control in A Batch Mixing TankDocument6 pagesThe CFD Simulation of Temperature Control in A Batch Mixing TankRodolfo BrandaoNo ratings yet
- Performance Analysis of Domestic RefrigeratorDocument10 pagesPerformance Analysis of Domestic RefrigeratorsyooloveNo ratings yet
- An Experimental Study of Temperature Distribution in An AutoclaveDocument13 pagesAn Experimental Study of Temperature Distribution in An AutoclaveKübraNo ratings yet
- On The Numerical and Experimental Study of Spray Cooling: M.R. Guechi, P.Desevaux and P. BaucourDocument11 pagesOn The Numerical and Experimental Study of Spray Cooling: M.R. Guechi, P.Desevaux and P. BaucourDibya SahooNo ratings yet
- Presentation 1Document36 pagesPresentation 1Rahul Tripathi50% (2)
- Flash Tank PDFDocument19 pagesFlash Tank PDFMufita RamadhinaNo ratings yet
- ME 315 - Heat Transfer Laboratory Experiment No. 5 Pool Boiling in A Saturated LiquidDocument10 pagesME 315 - Heat Transfer Laboratory Experiment No. 5 Pool Boiling in A Saturated LiquidmustafaNo ratings yet
- Direct Numerical Simulation of Subcooled Nucleate Pool BoilingDocument6 pagesDirect Numerical Simulation of Subcooled Nucleate Pool BoilingSreeyuth LalNo ratings yet
- Climbing Film Evaporator: I. CHE 1014 II. SB92Document14 pagesClimbing Film Evaporator: I. CHE 1014 II. SB92Khalid M MohammedNo ratings yet
- Heat Exchanger Report PDFDocument18 pagesHeat Exchanger Report PDFKeith QuinnNo ratings yet
- Refining of Oil Lab: Experiment NameDocument9 pagesRefining of Oil Lab: Experiment NameAmmar YasserNo ratings yet
- Princiapal Component Analysis of Petroleum SamplesDocument12 pagesPrinciapal Component Analysis of Petroleum SamplesJosef HmarNo ratings yet
- Application of COMSOL in Chemical EngineeringDocument11 pagesApplication of COMSOL in Chemical Engineeringjoseferrehigieneuru100% (1)
- Refining Technology Workbook Experiment 01Document4 pagesRefining Technology Workbook Experiment 01Muhammad MohtashimNo ratings yet
- Drawer DrierDocument10 pagesDrawer Driernn1129374No ratings yet
- CD4063Document25 pagesCD4063Mir HassanNo ratings yet
- Exp 07 SedimentationDocument13 pagesExp 07 SedimentationAmni SyazwaniNo ratings yet
- Turbine Blade Cooling by Air Using Different MethodsDocument22 pagesTurbine Blade Cooling by Air Using Different MethodsclaudiomctNo ratings yet
- An Engineering Model of Coils and Heat Exchangers For HVAC System Simulation and OptimizationDocument6 pagesAn Engineering Model of Coils and Heat Exchangers For HVAC System Simulation and Optimizationsilentsoldier781344No ratings yet
- Conventional MeasurementDocument10 pagesConventional MeasurementAna MoraNo ratings yet
- Fluorimetry AsignDocument7 pagesFluorimetry Asignshonu2009No ratings yet
- Disawas Experimental Investigation On The Performance of The Refrigeration Cycle Using A Two Phase Ejector As An Expansion Device 2004Document8 pagesDisawas Experimental Investigation On The Performance of The Refrigeration Cycle Using A Two Phase Ejector As An Expansion Device 2004Mohamed HassanainNo ratings yet
- Thermodynamic Modeling of Absorption Chiller PDFDocument10 pagesThermodynamic Modeling of Absorption Chiller PDFVaidyanathan KSNo ratings yet
- Rationally Based Model For Evaluating The Optimal Refrigerant Mass Charge in Refrigerating MachinesDocument15 pagesRationally Based Model For Evaluating The Optimal Refrigerant Mass Charge in Refrigerating MachinesDante Zamora MoscosoNo ratings yet
- Differential Scanning CalorimetryDocument7 pagesDifferential Scanning CalorimetryGintoki SakataNo ratings yet
- Analysis of Degradation Products by Thermal Volatilization Analysis at Subambient Temperatures (McNeill1977)Document12 pagesAnalysis of Degradation Products by Thermal Volatilization Analysis at Subambient Temperatures (McNeill1977)alberto.demarco.2No ratings yet
- Experimental Study AND Numerical Simulation OF Preform Infrared Radiative HeatingDocument8 pagesExperimental Study AND Numerical Simulation OF Preform Infrared Radiative HeatingRafael Calle Napoleon LuisNo ratings yet
- Wambui, Henrey (0386801) - ENGR - 4477 - Project Report PDFDocument15 pagesWambui, Henrey (0386801) - ENGR - 4477 - Project Report PDFStephanie BoatengNo ratings yet
- Summer Internship Report: Supervised By: Dr. Om Prakash Singh Iit-BhuDocument13 pagesSummer Internship Report: Supervised By: Dr. Om Prakash Singh Iit-Bhu2K18/PE/007 ANIKET GUPTANo ratings yet
- Refrigerant UnitDocument33 pagesRefrigerant UnitSiti ZulaihaNo ratings yet
- (Unit Operations Laboratory-2) : Name: Siraj Ali Aldeeb ID: 3214118Document11 pages(Unit Operations Laboratory-2) : Name: Siraj Ali Aldeeb ID: 3214118Siraj AL sharifNo ratings yet
- ThermoDocument12 pagesThermoTashi BestNo ratings yet
- Crystallization of Polypropylene at High Cooling Rates Microscopic and Calorimetric Studies 2012 Journal of Applied Polymer ScienceDocument14 pagesCrystallization of Polypropylene at High Cooling Rates Microscopic and Calorimetric Studies 2012 Journal of Applied Polymer ScienceLubomirBenicekNo ratings yet
- Characterization of Thermal Contact in Injection Molding Via The Combination of An Infrared Hollow Waveguide System and A Two-Thermocouple ProbeDocument7 pagesCharacterization of Thermal Contact in Injection Molding Via The Combination of An Infrared Hollow Waveguide System and A Two-Thermocouple ProbeHamed TouatiNo ratings yet
- Medidas de Temp en DevanadosDocument1 pageMedidas de Temp en DevanadosFernando LoteroNo ratings yet
- Minimize The Direct Sun Exposure and Heat Absorption (Shading)Document11 pagesMinimize The Direct Sun Exposure and Heat Absorption (Shading)Naveed Akhtar BalochNo ratings yet
- Heat Transfer in Freeze-Drying ApparatusDocument25 pagesHeat Transfer in Freeze-Drying ApparatusErickMartinS100% (1)
- Extended Surfaces Lab ReportDocument13 pagesExtended Surfaces Lab Reportabdulrehmanmani418383No ratings yet
- 5 Distillation Final ReportDocument7 pages5 Distillation Final ReportElzubair EljaaliNo ratings yet
- EVAPORATORDocument11 pagesEVAPORATOROssama BohamdNo ratings yet
- Thermal Analysis For Bulk-Micromachined Electrothermal Hydraulic Microactuators Using A Phase Change MaterialDocument9 pagesThermal Analysis For Bulk-Micromachined Electrothermal Hydraulic Microactuators Using A Phase Change MaterialAhmed Mohammed AwadNo ratings yet
- Roll-to-Roll Manufacturing: Process Elements and Recent AdvancesFrom EverandRoll-to-Roll Manufacturing: Process Elements and Recent AdvancesJehuda GreenerNo ratings yet
- 1.0 Intro To Aircon (Properties of Moist Air) With Sample ProblemDocument10 pages1.0 Intro To Aircon (Properties of Moist Air) With Sample ProblemRenneil De PabloNo ratings yet
- Valence Bond Theory - Chemistry LibreTextsDocument2 pagesValence Bond Theory - Chemistry LibreTextsguddanNo ratings yet
- G12 - Classification of MatterDocument32 pagesG12 - Classification of MatterChristian CatacutanNo ratings yet
- Instrumentation & Process ControlDocument51 pagesInstrumentation & Process ControlChiến Phan Công100% (1)
- MCQs of CMPDocument4 pagesMCQs of CMPHoomi ShbNo ratings yet
- Crystalliztion EquipmentDocument1 pageCrystalliztion EquipmentChem.EnggNo ratings yet
- ENGG1500 Study Guide S1 2018 PDFDocument137 pagesENGG1500 Study Guide S1 2018 PDFKatty TsaiNo ratings yet
- Electrostatic Precipitator (ESP)Document29 pagesElectrostatic Precipitator (ESP)yoga pratamaNo ratings yet
- Fe-Nb-Ni (Iron-Niobium-Nickel) : Binary SystemsDocument4 pagesFe-Nb-Ni (Iron-Niobium-Nickel) : Binary Systemsabdul basitNo ratings yet
- Effect of Capillary Tube Shapes On The Performance of Vapour Compression Refrigeration Cycle Using Nano-RefrigerantDocument10 pagesEffect of Capillary Tube Shapes On The Performance of Vapour Compression Refrigeration Cycle Using Nano-RefrigerantIJRASETPublicationsNo ratings yet
- Mass Transfer Coefficient: - The Values of The Coefficients Are Usually Reported As Correlations of Dimensionless NumbersDocument26 pagesMass Transfer Coefficient: - The Values of The Coefficients Are Usually Reported As Correlations of Dimensionless NumbersermiasNo ratings yet
- Material Science ..Crystal StructureDocument24 pagesMaterial Science ..Crystal StructureSajal MathurNo ratings yet
- Chemistry Why Do Liquids Boil at Different TemperaturesDocument3 pagesChemistry Why Do Liquids Boil at Different TemperaturesVALERY JOHANA RONCALLO BARRIOSNo ratings yet
- GATE Chemical Engineering 1994Document8 pagesGATE Chemical Engineering 1994Ishika PadhyNo ratings yet
- FSRU Toscana LNG and GAS Quality and Measurement ManualDocument29 pagesFSRU Toscana LNG and GAS Quality and Measurement Manualhikosko100% (1)
- Calculations 2Document3 pagesCalculations 2LeoNo ratings yet
- Properties of Metals: Metallic BondingDocument2 pagesProperties of Metals: Metallic BondingNuan Ting NgNo ratings yet
- EXPERIMENT 4 - Electrostatic PrecipitatorDocument19 pagesEXPERIMENT 4 - Electrostatic Precipitatorcoklatvanilla94No ratings yet
- Sampling Conditioning SystemDocument24 pagesSampling Conditioning System王祚No ratings yet
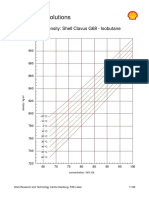
- Shell Global Solutions: Density: Shell Clavus G68 - IsobutaneDocument5 pagesShell Global Solutions: Density: Shell Clavus G68 - IsobutaneJavier AffifNo ratings yet
- Advance Refrigeration SystemDocument2 pagesAdvance Refrigeration SystemPunit ShindeNo ratings yet
- Innovative Vapor CompressionDocument13 pagesInnovative Vapor CompressionCj MoLanoNo ratings yet
- Experiment No. 4 (Viscosity)Document9 pagesExperiment No. 4 (Viscosity)Ranie MagpocNo ratings yet
- Gujarat Technological UniversityDocument2 pagesGujarat Technological UniversityKinnari PatelNo ratings yet
- Monthly Examination: Answer All The QuestionsDocument5 pagesMonthly Examination: Answer All The QuestionsJeevanandam ShanmugasundaramNo ratings yet
- Pipe Flow CalculationsDocument2 pagesPipe Flow CalculationsputrudeNo ratings yet
- Unit 5 Special Refrigeration SystemDocument93 pagesUnit 5 Special Refrigeration SystemNguyễn Văn Ngọc100% (1)
- Reservoir EngineeringDocument164 pagesReservoir EngineeringPhong NguyenNo ratings yet