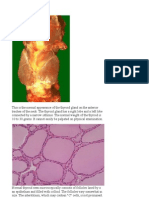
Professional Documents
Culture Documents
General Cytogenetics Information
General Cytogenetics Information
Uploaded by
jo_jo_maniaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
General Cytogenetics Information
General Cytogenetics Information
Uploaded by
jo_jo_maniaCopyright:
Available Formats
General Cytogenetics Information Basics
What Are Chromosomes?
Spanish Version
Cytogenetics is the study of chromosomes and the related disease states caused by abnormal chromosome number and/or structure. Chromosomes are complex structures located in the cell nucleus, they are composed of DNA, histone and nonhistone proteins, RNA , and polysaccharides. They are basically the "packages" that contain the DNA. Normally chromosomes can't be seen with a light microscope but during cell division they become condensed enough to be easily analyzed at 1000X. To collect cells with their chromosomes in this condensed state they are exposed to a mitotic inhibitor which blocks formation of the spindle and arrests cell division at the metaphase stage. A variety of tissue types can be used to obtain chromosome preparations. Some examples include peripheral blood, bone marrow, amniotic fluid, and products of conception. Although specific techniques differ according to the type of tissue used, the basic method for obtaining chromosome preparations is as follows: Sample log-in and initial setup. Tissue culture (feeding and maintaining cell cultures). Addition of a mitotic inhibitor to arrest cells at metaphase. Harvest cells. This step is very important in obtaining high quality preparations. It involves exposing the cells to a hypotonic solution followed by a series of fixative solutions. This causes the cells to expand so the chromosomes will spread out and can be individually examined. Stain chromosome preparations to detect possible numerical and structural changes.
Metacentric (Chromosome 1)
Chromosome Morphology
Under the microscope chromosomes appear as thin, thread-like structures. They all have a short arm and long arm separated by a primary constriction called the centromere. The short arm is designated as p and the long arm as q. The centromere is the location of spindle attachment and is an integral part of the chromosome. It is essential for the normal movement and segregation of chromosomes during cell division. Human metaphase chromosomes come in three basic shapes and can be categorized according to the length of the short and long
Submetacentric (Chromosome 9)
arms and also the centromere location. Metacentric chromosomes have short and long arms of roughly equal length with the centromere in the middle. Submetacentric chromosomes have short and long arms of unequal length with the centromere more towards one end. Acrocentric chromosomes have a centromere very near to one end and have very small short arms. They frequently have secondary constrictions on the short arms that connect very small pieces of DNA, called stalks and satellites, to the centromere. The stalks contain genes which code for ribosomal RNA. The diagrams to the left, called ideograms*, of G-banded chromosomes 1, 9, and 14 are typical examples of metacentric, submetacentric, and acrocentric chromosomes respectively. The ideogram is basically a "chromosome map" showing the relationship between the short and long arms, centromere (cen), and in the case of acrocentric chromosomes the stalks (st) and satellites (sa). The specific banding patterns are also illustrated. Each band is numbered to aid in describing rearrangements.
Acrocentric (Chromosome 14)
Chromosome Analysis
Virtually all routine clinical Cytogenetic analyses are done on chromosome preparations that have been treated and stained to produce a banding pattern specific to each chromosome. This allows for the detection of subtle changes in chromosome structure. The most common staining treatment is called Gbanding. A variety of other staining techniques are available to help identify specific abnormalities. Once stained metaphase chromosome preparations have been obtained they can be examined under the microscope. Typically 15-20 cells are scanned and counted with at least 5 cells being fully analyzed. During a full analysis each chromosome is critically compared band-for-band with it's homolog. It is necessary to examine this many cells in order to detect clinically significant mosaicism (see below). Following microscopic analysis, either photographic or computerized digital images of the best quality metaphase cells are made. Each chromosome can then be arranged in pairs according to size and banding pattern into a karyotype. The karyotype allows the Cytogeneticist to even more closely examine each chromosome for structural changes. A written description of the karyotype which defines the chromosome analysis is then made.
*Ideograms courtesy of Tim Knight, Fred Hutchinson Cancer Research Center, Seattle, WA.
Chromosome Abnormali ties
Normal Chromosomes
Normal human somatic cells have 46 chromosomes: 22 pairs, or homologs, of autosomes (chromosomes 1-22) and two sex chromosomes. This is called the diploid number. Females carry two X chromosomes (46,XX) while males have an X and a Y (46,XY). Germ cells (egg and sperm) have 23 chromosomes: one copy of each autosome plus a single sex chromosome. This is referred to as the haploid number. One chromosome from each autosomal pair plus one sex chromosome is inherited from each parent. Mothers can contribute only an X chromosome to their children while fathers can contribute either an X or a Y. Normal 550 and 650 Band Karyotypes
Chromosome Abnormalities
Although chromosome abnormalities can be very complex there are two basic types: numerical and structural. Both types can occur simultaneously. Numerical abnormalities involve the loss and/or gain of a whole chromosome or chromosomes and can include both autosomes and sex chromosomes. Generally chromosome loss has a greater effect on an individual than does chromosome gain although these can also have severe consequences. Cells which have lost a chromosome are monosomy for that chromosome while those with an extra chromosome show trisomy for the chromosome involved. Nearly all autosomal monosomies die shortly after conception and only a few trisomy conditions survive to full term. The most common autosomal numerical abnormality is Down Syndrome or trisomy-21. Trisomies for chromosomes 13 and 18 may also survive to birth but are more severely affected than individuals with Down Syndrome. Curiously, a condition called triploidy in which there is an extra copy of every chromosome (69 total), can occasionally survive to birth but usually die in the newborn period. Another general rule is that loss or gain of an autosome has more severe consequences than loss or gain of a sex chromosome. The most common sex chromosome abnormality is monosomy of the X chromosome (45,X) or Turner Syndrome. Another fairly common example is Klinefelter Syndrome (47,XXY). Although there is substantial variation within each syndrome, affected individuals often lead fairly normal lives. Occasionally an individual carries an extra chromosome
which can't be identified by it's banding pattern, these are called marker chromosomes. The introduction of FISH techniques has been a valuable tool in the identification of marker chromosomes. Structural abnormalities involve changes in the structure of one or more chromosomes. They can be incredibly complex but for the purposes of this discussion we will focus on the three of the more common types: Deletions involve loss of material from a single chromosome. The effects are typically severe since there is a loss of genetic material. Inversions occur when there are two breaks within a single chromosome and the broken segment flips 180 (inverts) and reattaches to form a chromosome that is structurally out-of-sequence. There is usually no risk for problems to an individual if the inversion is of familial origin (has been inherited from a parent.) There is a slightly increased risk if it is a de novo (new) mutation due possibly to an interruption of a key gene sequence. Although an inversion carrier may be completely normal, they are at a slightly increased risk for producing a chromosomally unbalanced embryo. This is because an inverted chromosome has difficulty pairing with it's normal homolog during meiosis, which can result in gametes containing unbalanced derivative chromosomes if an unequal cross-over event occurs. Translocations involve exchange of material between two or more chromosomes. If a translocation is reciprocal (balanced) the risk for problems to an individual is similar to that with inversions: usually none if familial and slightly increased if de novo. Problems arise with translocations when gametes from a balanced parent are formed which do not contain both translocation products. When such a gamete combines with a normal gamete from the other parent the result is an unbalanced embryo which is partially monosomic for one chromosome and partially trisomic for the other.
Numerical and structural abnormalities can be further divided into two main categories: constitutional, those you are born with; and acquired, those that arise as secondary changes to other diseases such as cancer. Sometimes individuals are found who have both normal and abnormal cell lines. These people are called mosaics and
in the vast majority of these cases the abnormal cell line has a numerical chromosome abnormality. Structural mosaics are extremely rare. The degree to which an individual is clinically affected usually depends on the percentage of abnormal cells. A routine Cytogenetic analysis typically includes the examination of at least 15-20 cells in order to rule out any clinically significant mosaicism. These are just some of the more common abnormalities encountered by a Cytogenetic Laboratory. Because the number of abnormal possibilities is almost infinite, a Cytogeneticist must be trained to detect and interpret virtually any chromosome abnormality that can occur.
Examples of Chromosome Abnormalities
Click on any of the following links to see some examples of numerical and structural chromosome abnormalities: Example 1: Down Syndrome, a common numerical abnormality. Example 2: An inversion in chromosome 10. Example 3: An interstitial deletion of chromosome 16. Example 4: A translocation between chromosomes 2 and 15. Example 5: A translocation between chromosomes 5 and 8. Example 6: A subtle inversion in chromosome 3. Example 7: An interstitial deletion of chromosome 7. Example 8: An unbalanced translocation between chromosomes 13 and 14.
Fluorescent IN SITU Hybridization (FISH)
Version
Spanish
Fluorescent IN SITU Hybridization (FISH) is a relatively new technology utilizing fluorescently labeled DNA probes to detect or confirm gene or chromosome abnormalities that are generally beyond the resolution of routine Cytogenetics. The sample DNA (metaphase chromosomes or interphase nuclei) is first denatured, a process that separates the complimentary strands within the DNA double helix structure. The fluorescently labeled probe of interest is then added to the denatured sample mixture and hybridizes with the sample DNA at the target site as it reanneals (or reforms itself) back into a double helix. The probe signal can then be seen through a fluorescent microscope and the sample DNA scored for the presence or absence of the signal.
Metaphase FISH
FISH can be used in metaphase cells to detect specific microdeletions beyond the resolution of routine Cytogenetics or identify extra material of unknown origin. It can also help in cases where it is difficult to determine from routine Cytogenetics if a chromosome has a simple deletion or is involved in a subtle or complex rearrangement. In addition, metaphase FISH can detect some of the specific chromosome rearrangements seen in certain cancers. The number of microdeletion syndromes diagnosed by FISH is expanding rapidly. The sensitivity of these tests in every case is better than routine Cytogenetics but depends on the particular syndrome. In some syndromes, the probe is specific for the defective gene as in Williams Syndrome where a deletion has been shown in the elastin gene in 96% of individuals with a firm diagnosis. In other syndromes such as Prader-Willi/Angelman, the etiology of the syndrome is heterogeneous and microdeletions compose only a portion of the cases (60%).
Microdeletion Syndromes Currently Diagnosable with FISH
Cri-du-Chat Miller-Dieker Syndrome Smith-Magenis Syndrome Steroid Sulfatase Deficiency DiGeorge/Velo-CardioFacial/CATCH-22/Shprintzen Syndrome Kallman Syndrome Williams Syndrome Wolf-Hirschhorn Prader-Willi/Angelman Syndrome
Interphase FISH
FISH can be used in interphase cells to determine the chromosome number of one or more chromosomes as well as to detect some specific chromosome rearrangements that are characteristic for certain cancers. The primary advantage of interphase FISH is that it can be performed very rapidly if necessary, usually within 24 hours, because cell growth is not required. A good example is the Aneuploid Screen test which is performed on amniotic fluid cells when there is a strong clinical indication for one of the common trisomies. The sample nuclei are denatured and hybridized with DNA probes for chromosomes 13, 18, 21, X, and Y and results usually obtained within 24 hours. Routine Cytogenetics is included with an Aneuploid Screen to confirm the results or detect any abnormalities not detected by interphase FISH.
Click on the following links to see some FISH examples:
Interphase FISH Examples
This is an example of the Aneuploid Screen test where interphase nuclei from amniotic fluid cells have been combined with DNA probes for chromosomes 13, 18, 21, X, and Y. The nucleus on the left has been hybridized to probes for chromosomes 13 (green), and 21 (red). The nucleus on the right has been hybridized to probes for chromosomes 18 (aqua), X (green), and Y (red). Since there are two signals each for chromosomes 13, 18, and 21 and one signal each for the X and Y chromosome this fetus is a normal male with respect to the Aneuploid Screen test.
This Aneuploid Screen test shows a female fetus with trisomy-21. The nucleus on the left has been hybridized to probes for chromosomes 13 (green), and 21 (red) and clearly has three red signals. The nucleus on the right has been hybridized to probes for chromosomes 18 (aqua), X (green), and Y (red). Since it has two green signals and no red signal it is female.
Metaphase FISH Examples
This is an example of a metaphase cell that has been hybridized with the probe for DiGeorge/Velo-Cardio-Facial/CATCH 22/Shprintzen Syndrome which is caused by a microdeletion on chromosome 22. The probe in this particular case is a dual-color mixture of two seperate probes for chromosome 22. The green signal is an internal control and is located at 22q13. It allows for quick identification of both #22 chromosomes. The red signal is located at the DiGeorge region at 22q11.2. Since both 22's have the red signal in this cell there is not a microdeletion within the DiGeorge region and this individual would not have DiGeorge Syndrome.
This metaphase has been hybridized with a "painting" probe for chromosome 4 which causes the entire chromosome to fluoresce. One chromosome 4 from this individual was abnormal but it was difficult to determine from routine Cytogenetics if it had a small terminal deletion at 4q or was the result of a more complex rearrangement. Since both 4's are fluorescent along their entire length and no fluorescent material is present on any other chromosome, this suggests that the abnormality is a small terminal deletion. The metaphase below is from the same individual and further confirms this diagnosis.
This metaphase from the same individual as the cell above has been hybridized with a probe for the terminal part of chromosome 4q. Since there is only one green signal this confirms that one chromosome 4 is missing material from the terminal end of 4q. This case is a good example of how routine Cytogenetics and FISH can be used together to accurately diagnose subtle chromosome abnormalities.
This is an example of a metaphase cell that has been hybridized with the probe for Steroid Sulfatase Deficiency which is caused by a microdeletion on the X chromosome. The probe in this particular case is a mixture of two seperate probes for the X chromosome, both red in color. The "X cen" probe signal is an internal control and is located at the X centromere. It allows for quick identification of the X chromosome(s). The "Xp22.3" probe signal is located at the Steroid Sulfatase region at Xp22.3. Since there are two X chromosomes and only one has the Steroid Sulfatase gene signal, this individual is a female carrier for Steroid Sulfatase Deficiency.
You might also like
- MCB 252 Final Exam Study GuideDocument62 pagesMCB 252 Final Exam Study GuideJay ZNo ratings yet
- NAP Gen. Circular 3 GRDS For LGUsDocument36 pagesNAP Gen. Circular 3 GRDS For LGUsquickmelt03No ratings yet
- 5 Cyto AbnormalDocument9 pages5 Cyto AbnormalMerli Ann Joyce CalditoNo ratings yet
- Pelvis and Perineum Clinical CorrelationDocument4 pagesPelvis and Perineum Clinical CorrelationKeesha Mariel AlimonNo ratings yet
- Pathology of NoaDocument164 pagesPathology of NoaAnonymous milwFDXNo ratings yet
- 1.1 Sensory-Motor Skills Involve The Process of Receiving InformationDocument5 pages1.1 Sensory-Motor Skills Involve The Process of Receiving InformationTshepo MolotoNo ratings yet
- Eppd-A Discourse CommunityDocument11 pagesEppd-A Discourse Communityapi-254655486No ratings yet
- Actinic Keratosis: (Aka Bowen's Disease)Document5 pagesActinic Keratosis: (Aka Bowen's Disease)fadoNo ratings yet
- Commensal AmoebaDocument2 pagesCommensal AmoebaCoy NuñezNo ratings yet
- Bacterial Infections of The SkinDocument9 pagesBacterial Infections of The Skinbeia21No ratings yet
- 2011 07 Microbiology Mycobacterium Skin InfectionDocument6 pages2011 07 Microbiology Mycobacterium Skin InfectionCristinaConcepcionNo ratings yet
- The Complement SystemDocument4 pagesThe Complement SystemExamville.com100% (1)
- Essential Update: FDA Approves First Test To Predict AKI in Critically Ill PatientsDocument5 pagesEssential Update: FDA Approves First Test To Predict AKI in Critically Ill PatientsRika Ariyanti SaputriNo ratings yet
- Chronic Inflammatory Dermatoses Inflammatory Blistering DisordersDocument4 pagesChronic Inflammatory Dermatoses Inflammatory Blistering DisordersspringdingNo ratings yet
- DermDocument10 pagesDermyassrmarwaNo ratings yet
- Nephrotic Syndrome WikipediaDocument10 pagesNephrotic Syndrome WikipediaJohn KevlarNo ratings yet
- Cycle CellDocument16 pagesCycle CellRohingya EnglishNo ratings yet
- Renal SyndromeDocument13 pagesRenal SyndromeAndreas KristianNo ratings yet
- University of Santo Tomas: Faculty of Pharmacy - Department of Medical TechnologyDocument7 pagesUniversity of Santo Tomas: Faculty of Pharmacy - Department of Medical TechnologyWynlor AbarcaNo ratings yet
- Abdominal Wall, Omentum, Mesentery, RetroperitoneumDocument6 pagesAbdominal Wall, Omentum, Mesentery, RetroperitoneumMon Ordona De GuzmanNo ratings yet
- The Immune System OhtDocument6 pagesThe Immune System OhtKa-Shun Leung100% (1)
- Hematology Abnormal White Blood CellDocument4 pagesHematology Abnormal White Blood CellBryan James LinNo ratings yet
- Anterior Abdominal WallDocument5 pagesAnterior Abdominal WallAnonymous 2TzM1ZNo ratings yet
- SYPHYLISDocument1 pageSYPHYLISkhadzxNo ratings yet
- Vertebral Artery DissectionDocument2 pagesVertebral Artery DissectionTom MallinsonNo ratings yet
- Cancer EpigeneticsDocument48 pagesCancer EpigeneticsIqra SultanNo ratings yet
- Screening Test For Phagocytic Engulfment: DiapedesisDocument2 pagesScreening Test For Phagocytic Engulfment: DiapedesisBianca ANo ratings yet
- Midterm Chapter7Document43 pagesMidterm Chapter7Frances FranciscoNo ratings yet
- Haematology SAQDocument16 pagesHaematology SAQPowell KitagwaNo ratings yet
- Chapter 3 Genetic VariationDocument21 pagesChapter 3 Genetic VariationAbdulkarimNo ratings yet
- Iron Metabolism: DR Mukhtiar BaigDocument58 pagesIron Metabolism: DR Mukhtiar BaigdrmukhtiarbaigNo ratings yet
- Goljan Live Notes Day 1Document5 pagesGoljan Live Notes Day 1Daniyal AzmatNo ratings yet
- Hemostasis and Thrombosis: OutlineDocument11 pagesHemostasis and Thrombosis: OutlineManila MedNo ratings yet
- Harrisons: Introduction To Infectious DiseasesDocument3 pagesHarrisons: Introduction To Infectious Diseasesapi-3704562No ratings yet
- (MICROA - 2.1) Myeloid Tissue HistologyDocument6 pages(MICROA - 2.1) Myeloid Tissue HistologyHenryboi CañasNo ratings yet
- CNS PathologyDocument126 pagesCNS PathologyMichael-John FayNo ratings yet
- Anti EpCAM Antibodies For Detection of Metastatic CarcinomaDocument6 pagesAnti EpCAM Antibodies For Detection of Metastatic CarcinomaIulia Alexandra PredaNo ratings yet
- 1 Ana Intro Finals September 16 LaygoDocument3 pages1 Ana Intro Finals September 16 LaygombdelenaNo ratings yet
- Patho CA - Acute PancreatitisDocument1 pagePatho CA - Acute PancreatitisKNo ratings yet
- Pathology B - Gastrointestinal Tract (Esguerra, 2015)Document18 pagesPathology B - Gastrointestinal Tract (Esguerra, 2015)Ars MoriendiNo ratings yet
- Module2 ImmunologyDocument33 pagesModule2 ImmunologyAygul RamankulovaNo ratings yet
- PathologyDocument30 pagesPathologyTenorski baritonNo ratings yet
- Skin PathogensDocument4 pagesSkin PathogensEhi EdialeNo ratings yet
- Anat 4.3 GIT Histo - ZuluetaDocument8 pagesAnat 4.3 GIT Histo - Zuluetalovelots1234No ratings yet
- Introduction To HaemostasisDocument18 pagesIntroduction To Haemostasiswatchme3No ratings yet
- ENDOCRINE PATHOLOGY WebpathDocument35 pagesENDOCRINE PATHOLOGY Webpathapi-3766657No ratings yet
- Lymphoma: Pro - Dr.Ahmed EisaDocument45 pagesLymphoma: Pro - Dr.Ahmed EisaOmar Mohammed100% (1)
- Para Compre 2Document17 pagesPara Compre 2serainie maiNo ratings yet
- Neuropathology: FK UisuDocument28 pagesNeuropathology: FK UisuAnggi WahyuNo ratings yet
- Patho Final Study GuideDocument55 pagesPatho Final Study GuideBritNo ratings yet
- Histology Viscus Flow ChartDocument1 pageHistology Viscus Flow ChartNaser Hamdi ZalloumNo ratings yet
- Cytogenetics Note PDFDocument14 pagesCytogenetics Note PDFMerjema Bahtanović100% (1)
- Structural Biology of HIVDocument31 pagesStructural Biology of HIVLaura TapiaNo ratings yet
- Casts inDocument1 pageCasts ingregoryvoNo ratings yet
- Cytogenetics Course PackDocument29 pagesCytogenetics Course Packanonymous squashNo ratings yet
- Gametogenesis and Fertilization - Bio 30 PDFDocument2 pagesGametogenesis and Fertilization - Bio 30 PDFJoseph Paguio100% (1)
- Leukemias & Lymphomas - HY USMLEDocument87 pagesLeukemias & Lymphomas - HY USMLEMatt McGlothlinNo ratings yet
- Small Intestine 01 PDFDocument9 pagesSmall Intestine 01 PDFfadoNo ratings yet
- Chapter 13 Neoplastic Proliferations of White CellsDocument16 pagesChapter 13 Neoplastic Proliferations of White CellsOmar100% (1)
- Renal BiopsyDocument53 pagesRenal Biopsybusiness onlyyouNo ratings yet
- Hereditary Spherocytosis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandHereditary Spherocytosis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Case Report: Pulmonary Sequestration: A Case Report and Literature ReviewDocument4 pagesCase Report: Pulmonary Sequestration: A Case Report and Literature Reviewjo_jo_maniaNo ratings yet
- Canadian Contraception ConsensusDocument14 pagesCanadian Contraception Consensusjo_jo_maniaNo ratings yet
- Donordarah PDFDocument1 pageDonordarah PDFjo_jo_maniaNo ratings yet
- Donor DarahDocument1 pageDonor Darahjo_jo_maniaNo ratings yet
- NEW Weekly Indra 04-08 July 2011Document13 pagesNEW Weekly Indra 04-08 July 2011jo_jo_maniaNo ratings yet
- AnyelirDocument2 pagesAnyelirjo_jo_maniaNo ratings yet
- Structure Content: 1. EXERCISE 1 (Skill 1-2) 2. Toefl Review Exercise (Skill 1-2) 3. Answer KeysDocument3 pagesStructure Content: 1. EXERCISE 1 (Skill 1-2) 2. Toefl Review Exercise (Skill 1-2) 3. Answer Keysjo_jo_mania0% (1)
- OrtoDocument61 pagesOrtojo_jo_maniaNo ratings yet
- International Centre For Eye Health Teaching Set 2 The Eye in Primary Health CareDocument25 pagesInternational Centre For Eye Health Teaching Set 2 The Eye in Primary Health Carejo_jo_maniaNo ratings yet
- Sunday, 26/09 /2010 PT/LN/MG:: Consultant DR Mas Putra SPMDocument6 pagesSunday, 26/09 /2010 PT/LN/MG:: Consultant DR Mas Putra SPMjo_jo_maniaNo ratings yet
- Migraine Prophylaxis: Pharmacotherapy PerspectivesDocument11 pagesMigraine Prophylaxis: Pharmacotherapy Perspectivesjo_jo_maniaNo ratings yet
- Arterial Blood Gas: DR - Made Widia, Sp.A (K)Document19 pagesArterial Blood Gas: DR - Made Widia, Sp.A (K)jo_jo_mania100% (1)
- UveaDocument43 pagesUveajo_jo_mania100% (1)
- Histamin Dan inDocument30 pagesHistamin Dan inNurul FadilaturrahmiNo ratings yet
- Dyslexia: Developmental Dyslexia Is ADocument5 pagesDyslexia: Developmental Dyslexia Is Ajo_jo_mania100% (1)
- X RaysDocument1 pageX Raysjo_jo_maniaNo ratings yet
- An Endometrial Is A Procedure To Remove A Small Sample of The Lining of The UterusDocument2 pagesAn Endometrial Is A Procedure To Remove A Small Sample of The Lining of The Uterusjo_jo_maniaNo ratings yet
- Strategic Managemment Perry Ring AMR Apr 1985Document12 pagesStrategic Managemment Perry Ring AMR Apr 1985Anderson De Oliveira ReisNo ratings yet
- Bawang Merah Bawang PutihDocument3 pagesBawang Merah Bawang Putihzulfarahma50% (2)
- Case Study 2Document3 pagesCase Study 2Shahaneh BalateroNo ratings yet
- The Nehru DynastyV1.1Document7 pagesThe Nehru DynastyV1.1das_ankita0111No ratings yet
- A Presentation Digital T.V-An Innovation by AirtelDocument19 pagesA Presentation Digital T.V-An Innovation by AirtelkdkripaNo ratings yet
- Shifting The Focus From Forms To Form in The EFL ClassroomDocument7 pagesShifting The Focus From Forms To Form in The EFL ClassroomNguyên KanNo ratings yet
- Japanese Greetings For EverydayDocument13 pagesJapanese Greetings For EverydayMarvin MayormenteNo ratings yet
- Sony Corporation-FinalDocument13 pagesSony Corporation-FinalLakshan WeerasekeraNo ratings yet
- The Pentatonic Scale Cheat Sheet The Pentatonic Way LockedDocument10 pagesThe Pentatonic Scale Cheat Sheet The Pentatonic Way LockedfreimannNo ratings yet
- Good Morning Text MessagesDocument7 pagesGood Morning Text MessagesStephan MilesNo ratings yet
- Curriculum Vitae - Eka Devi Wulandari (2019) PDFDocument3 pagesCurriculum Vitae - Eka Devi Wulandari (2019) PDFDipta VioniNo ratings yet
- 100 Best-Selling Cases: The Case For LearningDocument29 pages100 Best-Selling Cases: The Case For LearningSultan KhanNo ratings yet
- EMBA Applied Value Investing (Ajdler) SP2016Document3 pagesEMBA Applied Value Investing (Ajdler) SP2016darwin12No ratings yet
- Manual m2x3 Enm b11Document94 pagesManual m2x3 Enm b11pondokjituNo ratings yet
- MUMONKAN - All 48 Koans With CommentariesDocument22 pagesMUMONKAN - All 48 Koans With CommentariesAhmad AlhourNo ratings yet
- 2013 Psat NMSQT Student GuideDocument56 pages2013 Psat NMSQT Student Guideclementscounsel87250% (1)
- Hem Lab Manual DiffDocument9 pagesHem Lab Manual DiffFatima Mae LusanNo ratings yet
- Dissertation Carl Friedrich GaussDocument6 pagesDissertation Carl Friedrich GaussWriteMyPaperForMeIn3HoursUK100% (1)
- First Metro Investment Vs Este Del Sol Mountain Reserve Inc - 141811 - November 15, 2001 - JDocument9 pagesFirst Metro Investment Vs Este Del Sol Mountain Reserve Inc - 141811 - November 15, 2001 - JulticonNo ratings yet
- Review: I Agree Completely I Agree To Some Extent I Disagree CompletelyDocument1 pageReview: I Agree Completely I Agree To Some Extent I Disagree CompletelynaluzotanNo ratings yet
- Ind As 115 VS Ind As 18Document7 pagesInd As 115 VS Ind As 18Yogendrasinh RaoNo ratings yet
- Blasting in U/G Mines: Roof Contour Holes Roof Contour HolesDocument10 pagesBlasting in U/G Mines: Roof Contour Holes Roof Contour HolesSiddharth MukhillaNo ratings yet
- A Spatial Analysis of Crop-Land Suitability For Sustainable Agricultural Development in Aiyar Basin, Tamil NaduDocument12 pagesA Spatial Analysis of Crop-Land Suitability For Sustainable Agricultural Development in Aiyar Basin, Tamil NaduBala Subramani KNo ratings yet
- Uea Coursework Word CountDocument4 pagesUea Coursework Word Counttgpazszid100% (2)
- Self Worth SpeechDocument1 pageSelf Worth SpeechAbigail Fritz GoloNo ratings yet
- Computer Notes - Scan ConversionDocument7 pagesComputer Notes - Scan Conversionecomputernotes100% (1)
- The Information Apocalypse Rough Draft-1Document6 pagesThe Information Apocalypse Rough Draft-1api-425080738No ratings yet