Professional Documents
Culture Documents
General Series Orders
General Series Orders
Uploaded by
DEEPAK KUMAR MALLICKOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
General Series Orders
General Series Orders
Uploaded by
DEEPAK KUMAR MALLICKCopyright:
Available Formats
General Series Orders (Chemistry Fact Sheet 3)
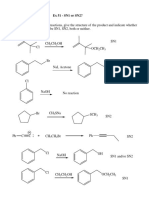
General series 1. Order I > II > III Why ? There is intermolecular Hbonding I. III has weak force of attraction and is most volatile. Intramolecular Hbonding in o-isomer makes it more volatile. CHO group is easily oxidised compared to keto group due to redusing hydrogen.
2.
B.P. of o, m, p-nitro phenol
o<m<p
Reactivity of ... with Tollens reagent 3. I > II > IV > III
4.
Reactivity of ... with Fehlings solution
I > II > IV > III
do
Extent of hydration of 5. I < II < III < IV
Aldehydes are more hydrated than ketones. Halide makes C of carbonyl group more electropositive.
Electrophilic nature of ........ for nucleophilic attack 6. I > II > III
CH3 group decreases +ve charge on C hence nucleophilic attack.
7.
Reactivity of isomeric 1, 2, 3 butyl halide towards elimination (E1 or E2)
3 < 2 < 1
due to stability of intermediate carbocation
Dehydration of 8. IV < I < II < III
Alcohol leading to increase in conjugation due to dehydration is more easily dehydration is more easily dehydrated. IV is vinylic, hence least.
9.
Stability of
Substituted alkenes are more stable.More the alkyl groups are I < II < III < IV attached to the doubly < V < VI bonded carbon atom more is the stability.
Stability of 10. I < III < II
II is more substituted than III (More hyperconjugation more stability)
Stability of 11. III > II > I > IV IV is vinylic while in conjugative, II allylic.
Stability of 12. I < IV < II < III III is 3 allylic and II is 1 allylic
13.
Dehydration of 1, 2, 3 isomeric butyl alcohol Boiling points of
3 < 2 < 1
More the stability of intermediate, greater the reactivity of chemical reaction. I, II have H-bonding but electronegativity of O > N hence Hbonding in II > I
14.
II > I > III
Formation of 15.
greater the stability, I > II > III > IV easier the formation (easiest I) of perticular species.
16.
Reactivity of CH bond (abstraction of H)
I < II < III < IV Vinyl < methyl 1 < 2 < V < VI < 3 < allylic
Leaving nature (tendency) of ... in SN reaction. 17.
If acid is strong, its I < II < III ~ IV conjugate base is < V < VI < VII weak and greater the < VIII leaving tendency.
Rate of esterification of the following acids with MeOH 18.
As the size of the substituents on the C increases, the I > II > III > IV tetrahedrally bonded >V interme. diate becomes more crowded and these slower the rate.
19.
Relative reactivity of ... with electrophile in SE reaction
I > II > IV > III > V
CH3 is o-, p-directing and responsible for activation.
Relative reactivity of these compunds with electrophile inSE reaction 20. II > I > III > IV
CH3 is o-, p-directing due to activation while COOH is mdirecting and deactivating group.
Relative reactivity of ... with electrophile in SE reaction. 21. II > I > IV > II
Activating effects of the following o, pdirectors. 22. II > I > III
As the number of sp3 hybridised C atoms separating the ring from the positively charged substituent increases, deactivating effect decreases due to less electronegativity. is best able to donate electrons there by giving a very stable uncharged intermediate. In cross conjugation diminished its ability to donate electrons to an arenium ion. Intermediates are benzylic cations. So CH3O(electron repelling) gives greater stability through delocalisation while NO2 (electron attracting) decreases stability. SN1 : 1 < 2 < 3 alkyl halide SN2 : 3 < 2 < 1 alkyl halide NO2 deactivates benzene ring for SE
Relative reactivity of ... towards SN1 reaction 23. II > I > III
Relative reactivity of ... towards SN1 and SN2 reaction 24.
SN1 : III > II > I SN2 : II < II < I
25.
Relative reactivity of ... with E+ (electrophile) in SE reaction.
II > I > III
26.
Order of SN2 reactivity of alkoxide nucleophiles SN2 reactivity is suseptible to steric I < IV < V < III hindrance by the < II nucleophile as well as by the size of alkyl group.
You might also like
- Ib Chemistry SL Chapter 10 PDF FreeDocument21 pagesIb Chemistry SL Chapter 10 PDF FreeJustin MatthewNo ratings yet
- 2010 A Level H2 P3 Suggested AnswersDocument10 pages2010 A Level H2 P3 Suggested AnswersMichelle LimNo ratings yet
- D 5092 - 04 - RduwotiDocument16 pagesD 5092 - 04 - RduwotiDEEPAK KUMAR MALLICKNo ratings yet
- CH-10 Halogen DerivativeDocument9 pagesCH-10 Halogen DerivativeKirtan Singh RaoNo ratings yet
- Haloalkanes and Haloarenes-Imp QNSDocument3 pagesHaloalkanes and Haloarenes-Imp QNSjamesNo ratings yet
- Reasoning Questions From Organic Chemistry by Manoj Kumar KV KishtwarDocument5 pagesReasoning Questions From Organic Chemistry by Manoj Kumar KV KishtwarShivesh Singh100% (1)
- Inorganic ChemistryDocument22 pagesInorganic ChemistryuniquestarNo ratings yet
- Alchohols PhenolsDocument4 pagesAlchohols PhenolsNikita's worldNo ratings yet
- Chemistry of BORON and SILICONDocument32 pagesChemistry of BORON and SILICONprashantNo ratings yet
- Srmeee Chemistry Modal PaperDocument10 pagesSrmeee Chemistry Modal PaperSubir Nath BhowmikNo ratings yet
- Reaction IntermediateDocument18 pagesReaction IntermediateManaswini P100% (1)
- Halo NewDocument10 pagesHalo NewMohammed IliasNo ratings yet
- Organic ChemistryDocument25 pagesOrganic ChemistryhimanisinghbasnalNo ratings yet
- Some Important Reasoning Based Questions of Organic ChemistryDocument17 pagesSome Important Reasoning Based Questions of Organic ChemistrySourajit Mukherjee100% (1)
- Reasoning Organic ChemDocument12 pagesReasoning Organic ChemUtkarsh BajpaiNo ratings yet
- NCERT Exemplar For Class 11 Chemistry Chapter 13 - Hydrocarbons (Book Solutions)Document27 pagesNCERT Exemplar For Class 11 Chemistry Chapter 13 - Hydrocarbons (Book Solutions)jackdish18No ratings yet
- The Reaction Gives Pure Alkyl HalidesDocument8 pagesThe Reaction Gives Pure Alkyl HalidesMohammed IliasNo ratings yet
- Reasoning Based Questions (6 Marks) Section-ADocument4 pagesReasoning Based Questions (6 Marks) Section-ARishi SharmaNo ratings yet
- Organic Reasoning NewDocument27 pagesOrganic Reasoning NewJeyanthiNo ratings yet
- Organozinc CompoundDocument8 pagesOrganozinc Compoundamanuel tafeseNo ratings yet
- Reasoning Ques in Organic ChemistryDocument14 pagesReasoning Ques in Organic ChemistryRIHINBHATNAGAR50% (2)
- CH 7 S Cbse BDocument13 pagesCH 7 S Cbse BjsjsjskakkakoNo ratings yet
- JF PVQQ FRFFNC Eu HCMGAMDocument67 pagesJF PVQQ FRFFNC Eu HCMGAMelonmuskonmoonNo ratings yet
- Ist Assignment On Amine 1.12.22Document7 pagesIst Assignment On Amine 1.12.22Sprabhu ParamNo ratings yet
- XII Organic Reasoning QuestionsDocument7 pagesXII Organic Reasoning Questionslakshvanthbala100% (1)
- Haloalkanes and Haloarenes Worksheet 16 With SolutionsDocument13 pagesHaloalkanes and Haloarenes Worksheet 16 With Solutionsvircritharun718No ratings yet
- Namma Kalvi 12th Chemistry Pta Question Papers 217338 PDFDocument68 pagesNamma Kalvi 12th Chemistry Pta Question Papers 217338 PDFmnareshg007No ratings yet
- Haloalkanes and Arenes - Q & ADocument10 pagesHaloalkanes and Arenes - Q & AcynicalNo ratings yet
- Reasoning Questions Organic Chemistry Class XiiDocument6 pagesReasoning Questions Organic Chemistry Class XiipreitaphilenaNo ratings yet
- Alcohol, Phenols and Ethers PDFDocument13 pagesAlcohol, Phenols and Ethers PDFRahul JaiswalNo ratings yet
- Reasoning Qns With Answers - Haloalkanes&Haloarenes 2Document8 pagesReasoning Qns With Answers - Haloalkanes&Haloarenes 2tanishka0307No ratings yet
- General Organic Chemistry: (A) Inductive EffectDocument44 pagesGeneral Organic Chemistry: (A) Inductive EffectHarsh TyagiNo ratings yet
- Lecture 16Document5 pagesLecture 16shashanebonnitaNo ratings yet
- First Term Exam Chemistry Answer KeyDocument9 pagesFirst Term Exam Chemistry Answer KeyRutujaNo ratings yet
- Acidic Powers & Their Orders (Chemistry Fact Sheet-1) : Acidic Power Order Why ?Document2 pagesAcidic Powers & Their Orders (Chemistry Fact Sheet-1) : Acidic Power Order Why ?DEEPAK KUMAR MALLICKNo ratings yet
- Haloalkanes and Haloarenes QDocument35 pagesHaloalkanes and Haloarenes QAbhinav BishtNo ratings yet
- XII B PB-1 Solution-1100729Document8 pagesXII B PB-1 Solution-1100729ASM CHENo ratings yet
- Conjugation: A Series of Overlapping P-Orbitals: Vinyl Carbon (SP Hybridized) Vinyl HydrogenDocument25 pagesConjugation: A Series of Overlapping P-Orbitals: Vinyl Carbon (SP Hybridized) Vinyl Hydrogenailimillah948No ratings yet
- Aldehyde and Ketones ReasonsDocument9 pagesAldehyde and Ketones ReasonsNandita KrishnanNo ratings yet
- Preboard - 3 ChemistryDocument3 pagesPreboard - 3 ChemistryvaniNo ratings yet
- Chemistry: A Free Web Support in EducationDocument4 pagesChemistry: A Free Web Support in EducationAshok PradhanNo ratings yet
- Final Testament-ChemistryDocument8 pagesFinal Testament-ChemistryJaysukh yt (Jay)No ratings yet
- Chemistry Class Xii Sample Paper 01 AnswersDocument8 pagesChemistry Class Xii Sample Paper 01 Answerssouparnikar1No ratings yet
- 13,14 Group TheoryDocument29 pages13,14 Group TheoryAlkaChoudharyNo ratings yet
- Chemistry 2014Document103 pagesChemistry 2014Godfred EkyeNo ratings yet
- CBSE Class 11 and 12 Chemistry Notes The P-Block ElementsDocument45 pagesCBSE Class 11 and 12 Chemistry Notes The P-Block ElementsPrabhuPalanichamy50% (2)
- 11 P Block Elements Study NotesDocument22 pages11 P Block Elements Study NotesVivek KumarNo ratings yet
- Chennaipublicschool: Holiday Home WorkDocument5 pagesChennaipublicschool: Holiday Home Workunapologeticreader007No ratings yet
- Ate Complexes of Secondary Boronic Esters As Chiral Organometallic-Type Nucleophiles For Asymmetric SynthesisDocument4 pagesAte Complexes of Secondary Boronic Esters As Chiral Organometallic-Type Nucleophiles For Asymmetric SynthesisludoNo ratings yet
- CHEM1202 - Revision PapersDocument12 pagesCHEM1202 - Revision PapersZANo ratings yet
- Class XII - All India Chemistry - Set-2: Cell NDocument6 pagesClass XII - All India Chemistry - Set-2: Cell NShashank ShekharNo ratings yet
- P-Block ElementsDocument17 pagesP-Block ElementsStuti TanwarNo ratings yet
- Class 12 Chemistry Important 5 Marks Questions With Answers PDFDocument29 pagesClass 12 Chemistry Important 5 Marks Questions With Answers PDFArpit KumarNo ratings yet
- GOC (Hints)Document2 pagesGOC (Hints)hchawla421No ratings yet
- DAz I0 XACIl 8 y RGFmy FNTDocument5 pagesDAz I0 XACIl 8 y RGFmy FNTAditya YadavNo ratings yet
- Haloalkanes and Haloarenes - CBSE Ex-3 Sol FileDocument5 pagesHaloalkanes and Haloarenes - CBSE Ex-3 Sol FileAyushNo ratings yet
- Xi Chem SP Set 1 MsDocument5 pagesXi Chem SP Set 1 MsAnanya AryaNo ratings yet
- Chemistry ExDocument12 pagesChemistry ExAmit KingNo ratings yet
- Reasoning (Ch-10) ChemistryDocument5 pagesReasoning (Ch-10) ChemistryKailash Singh ChauhanNo ratings yet
- Organic Reaction Mechanisms 1982: An annual survey covering the literature dated December 1981 through November 1982From EverandOrganic Reaction Mechanisms 1982: An annual survey covering the literature dated December 1981 through November 1982A. C. KnipeNo ratings yet
- Organic Reaction Mechanisms 1981: An annual survey covering the literature dated December 1980 through November 1981From EverandOrganic Reaction Mechanisms 1981: An annual survey covering the literature dated December 1980 through November 1981A. C. KnipeNo ratings yet
- Progress in Inorganic ChemistryFrom EverandProgress in Inorganic ChemistryKenneth D. KarlinNo ratings yet
- Fdot bb896 RPT PDFDocument178 pagesFdot bb896 RPT PDFDEEPAK KUMAR MALLICKNo ratings yet
- Abstract Design Mix m30Document2 pagesAbstract Design Mix m30DEEPAK KUMAR MALLICKNo ratings yet
- 4078Document11 pages4078Krishnakumar TodarmalNo ratings yet
- Is 4434 1978 PDFDocument18 pagesIs 4434 1978 PDFDEEPAK KUMAR MALLICKNo ratings yet
- 057A - Quotation - SOIL INVESTIGATION - ORANGE - GUJRAT - 04.08.16 PDFDocument3 pages057A - Quotation - SOIL INVESTIGATION - ORANGE - GUJRAT - 04.08.16 PDFDEEPAK KUMAR MALLICKNo ratings yet
- Is 2825 1969Document286 pagesIs 2825 1969DEEPAK KUMAR MALLICKNo ratings yet
- Hydraulic ConductivityDocument40 pagesHydraulic ConductivityDEEPAK KUMAR MALLICKNo ratings yet
- Silver Schmidt SF E 04.2013 LowDocument4 pagesSilver Schmidt SF E 04.2013 LowDEEPAK KUMAR MALLICKNo ratings yet
- Author: Same As of "Kaane - in - Iit - Wav"Document1 pageAuthor: Same As of "Kaane - in - Iit - Wav"DEEPAK KUMAR MALLICKNo ratings yet
- A Chart Comparing The SN1 Vs SN2 Reactions: That Can Prevent This Reaction From Occurring?Document3 pagesA Chart Comparing The SN1 Vs SN2 Reactions: That Can Prevent This Reaction From Occurring?মেঘলা আকাশNo ratings yet
- Edexcel IAL Unit 4 ChemDocument190 pagesEdexcel IAL Unit 4 Chemthisumisg2007No ratings yet
- Organic Chemistry Question PaperDocument2 pagesOrganic Chemistry Question PaperMOHAMED HISHAMNo ratings yet
- Alkyl HalidesDocument87 pagesAlkyl HalidesDidi LeraNo ratings yet
- RM 1 Mix QUESTION 1 230418 201559Document7 pagesRM 1 Mix QUESTION 1 230418 201559Jash ShahNo ratings yet
- Structural Effects of ReactivityDocument4 pagesStructural Effects of ReactivityMarivic BarandaNo ratings yet
- Full Download Ebook Ebook PDF Organic Chemistry 12th Edition by T W Graham Solomons PDFDocument41 pagesFull Download Ebook Ebook PDF Organic Chemistry 12th Edition by T W Graham Solomons PDFjanet.martino412100% (43)
- Homework and Practice Q Chapter 11Document23 pagesHomework and Practice Q Chapter 11mak100% (1)
- Ex 51 - SN1 or SN2? Question OneDocument2 pagesEx 51 - SN1 or SN2? Question OneVarokah VarNo ratings yet
- Chemical Reactions: Lecture by Kalpajyoti Dihingia Department of Chemistry DHSK CollegeDocument21 pagesChemical Reactions: Lecture by Kalpajyoti Dihingia Department of Chemistry DHSK CollegeKalpa DihingiaNo ratings yet
- Spotlight - Phase-2 - (2022-23) - Week-1 - Paper-1 - Compile (2020-P-1) - (Only Que.)Document15 pagesSpotlight - Phase-2 - (2022-23) - Week-1 - Paper-1 - Compile (2020-P-1) - (Only Que.)Suryansh DummyNo ratings yet
- Chem 212 Alkyl Halide Problems 2Document1 pageChem 212 Alkyl Halide Problems 2kevinamy100% (1)
- F.Y. B. Pharmacy Human Anatomy and Physiology - I: Time: 3 Hours) (Max. Marks: 75 Instructions To The CandidatesDocument56 pagesF.Y. B. Pharmacy Human Anatomy and Physiology - I: Time: 3 Hours) (Max. Marks: 75 Instructions To The CandidatesKaif KhanNo ratings yet
- Ch07 LectureDocument87 pagesCh07 Lectureshantalle gabrielNo ratings yet
- CHEM 215 F12 Chapter 13 Notes UMICHDocument13 pagesCHEM 215 F12 Chapter 13 Notes UMICHRoxanne IlaganNo ratings yet
- Chapter7elimination Ans SubstnDocument22 pagesChapter7elimination Ans Substnjagabandhu_patraNo ratings yet
- Nucleophilic Substitution Reaction (SN2) - 1Document19 pagesNucleophilic Substitution Reaction (SN2) - 1Ramesh KatkamNo ratings yet
- Pre Board Exam, 2020-21 Chemistry, (043) Theory Class - XIIDocument8 pagesPre Board Exam, 2020-21 Chemistry, (043) Theory Class - XIIKshreeNo ratings yet
- Roman A. Valiulin - Organic Chemistry - 100 Must-Know Mechanisms-Walter de Gruyter (2020)Document250 pagesRoman A. Valiulin - Organic Chemistry - 100 Must-Know Mechanisms-Walter de Gruyter (2020)Daniel Arias100% (1)
- CHEM 203 Midterm Exam 2Document7 pagesCHEM 203 Midterm Exam 2pNo ratings yet
- Synthesis of 1-Bromobutane From 1-ButanolDocument11 pagesSynthesis of 1-Bromobutane From 1-ButanolAlice Nguyen50% (2)
- Cellulose DerivativesBDocument17 pagesCellulose DerivativesBGina Lugina Aprilina100% (2)
- Chem 232 Fall 2015 UIUC NotesDocument274 pagesChem 232 Fall 2015 UIUC NotesSri KondabattulaNo ratings yet
- Chapter 7Document189 pagesChapter 7Eshita SharmaNo ratings yet
- Substitution Vs Elimination Practice Problems 3 Answer Key Rev42213 PDFDocument3 pagesSubstitution Vs Elimination Practice Problems 3 Answer Key Rev42213 PDFsamfarmer333100% (1)
- Chapter 19 Ver 1Document101 pagesChapter 19 Ver 1jsaddsa jsasdNo ratings yet
- XII Reagents and Mechanism LDADocument21 pagesXII Reagents and Mechanism LDAAmey PatneNo ratings yet
- Unit 06Document9 pagesUnit 06abhilashNo ratings yet
- HalogenoalkanesDocument16 pagesHalogenoalkaneskudec2008No ratings yet